Novel cannabinoid receptor ligands, pharmaceutical compositions containing them, and process for their preparation
A compound and stereoisomer technology, applied in the field of cannabinoid receptor modulators, can solve problems such as poor compliance and liver toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
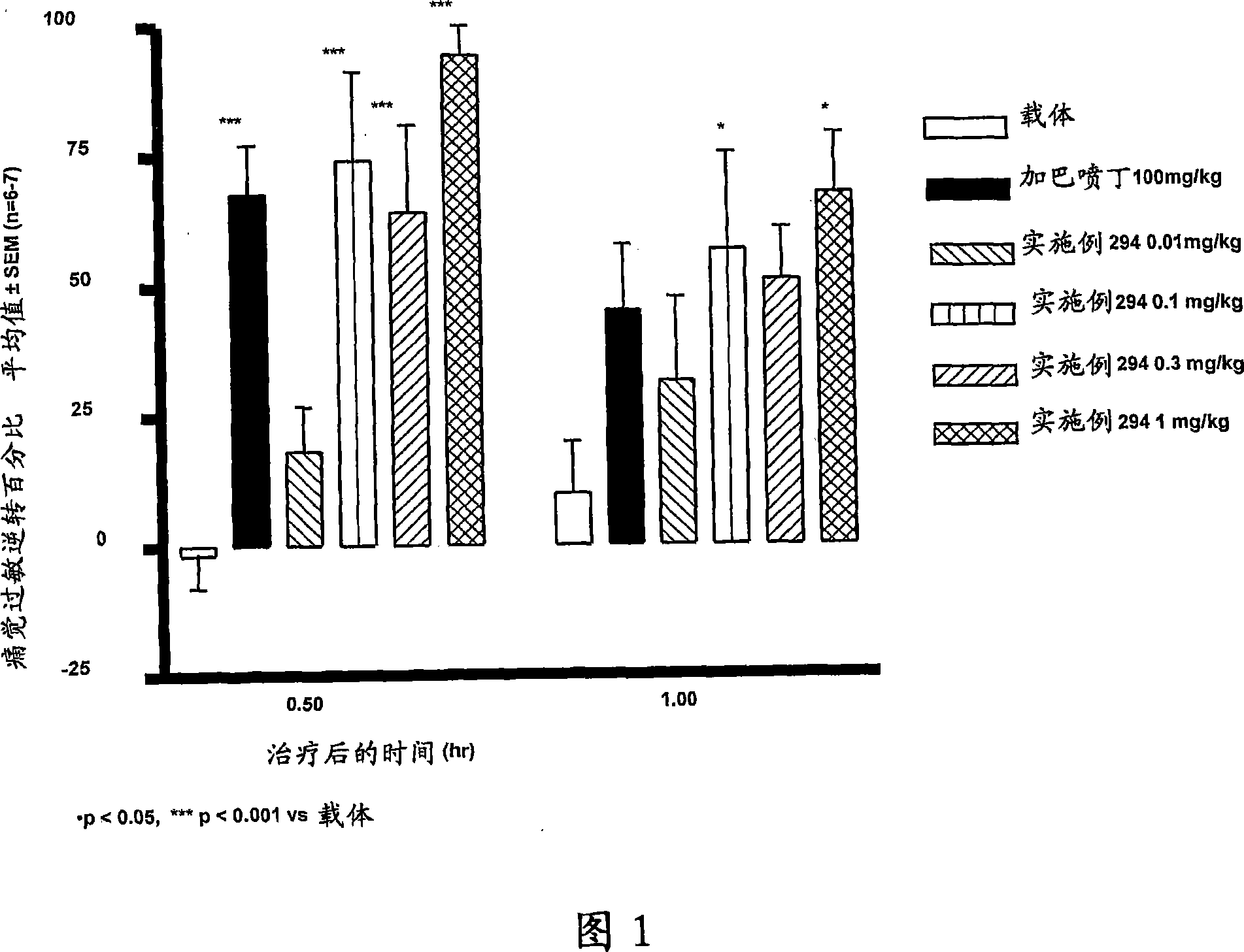
Image
Examples
Embodiment 101
[0777] N(7)-piperidin-1-yl-5-(2-bromophenyl)-5,6-diazatetracyclo[7.3.1.1 3,11 .0 4,8 ] Fourteen carbon-4(8),6-diene-7-carboxamide
[0778] The solution of Intermediate 1 (300mg, 0.78mmol) in DMF (3ml) was used at room temperature with BOP reagent (319mg, 0.72mmol) and Et 3 N (0.10 ml, 0.99 mmol) was treated for 15 minutes, after which, N-aminopiperidine (80 μl, 0.90 mmol) was added to the mixture and stirred at room temperature for 1 hour. The mixture was poured into water, the formed precipitate was collected by filtration, dried and purified by flash silica gel chromatography to obtain the pure title compound (270 mg, 74%). M.P.: 244°C. 1H-NMR(δppm, CDCl 3 , 300MHz): 7.72 (d, J = 7.6, 1H); 7.65 (br.s, 1H); 7.50-7.30 (m, 3H; 3.97 (br.s, 1H); 2.85 (br.s, 4H); 2.51 (br.s, 1H); 2.16 (br.s, 2H); 2.10-1.50 (m, 14H); 1.40 (br.s, 2H). IR (cm -1 , KBr): 3311(w), 2913(s), 2844(m), 2793(m), 1687(s), 1570(w), 1522(s), 1489(m), 1479(m), 1440 (m), 1352(m), 1227(m), 1214(m). MS(m / z)469.4([M+H1...
Embodiment 102
[0780] N(7)-Benzyl-5-(2-bromophenyl)-5,6-diazatetracyclo[7.3.1.1 3,11 .0 4,8 ] Fourteen carbon-4(8),6-diene-7-carboxamide
[0781] The title compound was synthesized by a method similar to that described in Example 101. Use Intermediate 1 (300mg, 0.78mmol), DMF (3ml), Et 3 N (0.10ml, 0.99mmol), BOP reagent (319mg, 0.72mmol) and benzylamine (80μl, 0.72mmol) gave the title compound (280mg, 76%). M.P.: 201°C. 1 H-NMR(δppm, CDCl 3 , 300MHz): 7.70 (d, J=7.5, 1H); 7.44 (dd, J=7.5, 1.5, 1H); 7.41-7.20 (m, 8H); 4.63 (dd, J=14.4, 6.0, 1H); 4.50 (dd, J=16.5, 5.4, 1H); 4.00 (br.t, J=5.1, 1H); 2.52 (br.t, J=5.1, 1H); 2.13 (br.s, 2H); 2.13 1.92 (m, 4H); 1.92-1.65 (m, 6H). IR(cm -1 , KBr): 3428(m), 2908(s), 2845(m), 1672(s), 1566(m), 1522(s), 1498(s), 1472(s), 1351(m), 1087 (m), 1024 (m), 1010 (m), 778 (m), 763 (m), 726 (m), 698 (m). MS(m / z): 476.4([M+H] + ).
Embodiment 103
[0783] N(7)-morpholino-5-(4-chlorophenyl)-5,6-diazatetracyclo[7.3.1.1 3,11 .0 4,8 ] Fourteen carbon-4(8),6-diene-7-carboxamide
[0784] The title compound was synthesized by a method similar to that described in Example 101. Use Intermediate 2 (100mg, 0.29mmol), DMF (1.0ml), Et 3 N (40 μl, 0.29 mmol), BOP reagent (129 mg, 0.29 mmol) and N-aminomorpholine (28 μl, 0.29 mmol) gave the title compound (97 mg, 78%). M.P.: 220°C. 1 H-NMR(δppm, CDCl 3 , 300MHz): 7.75 (br.s, 1H); 7.47 (d, J = 8.1, 2H); 7.29 (d, J = 8.1, 2H); 3.96 (br.s, 1H); 3.85 (t, J = 4.5, 4H); 2.99 (br.s, 1H); 2.95 (t, J=4.5, 5H); 2.20 (br.s, 2H); 2.10-1.76 (m, 10H). IR(KBr, cm -1 ): 2915(s), 2848(m), 1674(s), 1532(m), 1498(s), 1304(m), 1267(m), 1219(m), 1112(s), 1091(s) ), 895(m), 838(s). MS(m / z): 427.3([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com