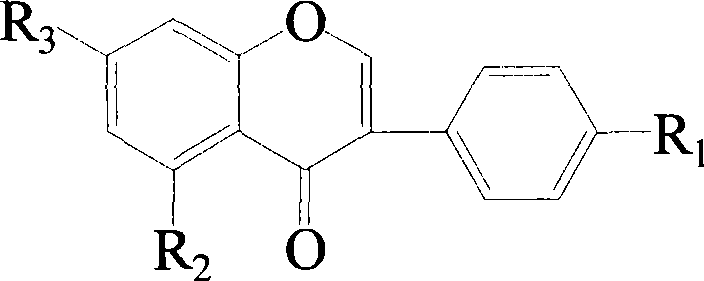
Dyestuff lignin sulfonic acid ester derivatices and preparation method thereof
A technology for sulfonic acid esters and derivatives, applied in the field of genistein sulfonic acid ester derivatives and their preparation, can solve the problems of poor water solubility and fat solubility of genistein, unclear application prospects, successful anti-cancer transformation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
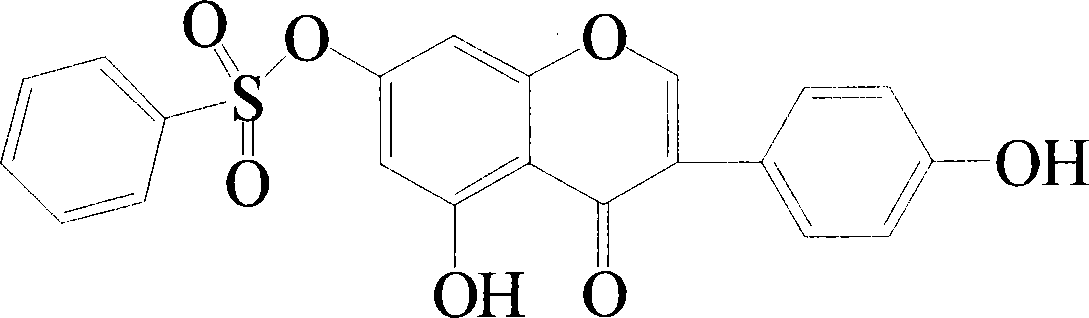
[0034] Example 1 Compound 4', the synthesis of 5-dihydroxyl-7-benzenesulfonyloxy isoflavone
[0035] Disperse genistein (0.4mmol, 0.108g) in 10mL of dichloromethane, add 0.01g of potassium tert-butoxide, and slowly add 0.48mmol (0.0847g) of benzenesulfonyl chloride dropwise at -20°C under the protection of Ar . Incubate for 0.5-24 hours. Filter the mixture, spin the filtrate to dryness under reduced pressure, and purify by column chromatography (chloroform / acetone=10:1) to obtain a pure product.
[0036] 1 H NMR (400MHz, CDCl 3 ): δ12.7 (1H, s, 5-OH), 7.94 (1H, s, 2-H), 7.90 (2H, d, J=7.7Hz, 2”, 6”-benH), 7.72 (1H, t, J=7.5Hz, 4"-H), 7.59 (2H, t, J=7.7Hz, 3", 5"-benH), 7.39 (2H, d, J=8.5Hz, 2', 6'- ArH), 6.90 (2H, d, J=8.5Hz, 3', 5'-ArH), 6.77 (1H, d, 4 J=2.0Hz, 8-ArH), 6.39 (1H, d, 4 J=2.0Hz, 6-ArH), 5.39 (1H, s, 4'-OH); m / z (EI) 411.08 (M + +1, 100%).
Embodiment 2
[0037] Example 2 Compound 5-dihydroxyl-4', the synthesis of 7-diphenylsulfonyloxy isoflavone
[0038] Genistein (0.4mmol, 0.108g) was dispersed in 10mL of dichloromethane, 0.01g of KOH was added, and 0.96mmol (0.1694g) of benzenesulfonyl chloride was slowly added dropwise at -20°C under the protection of Ar. Incubate for 0.5 to 24 hours. Filter the mixture, spin the filtrate to dryness under reduced pressure, and purify by column chromatography (chloroform / acetone=20:1) to obtain a pure product.
[0039] 1 H NMR (400MHz, CDCl 3 ): δ12.7 (1H, s, 5-OH), 7.95 (1H, s, 2-H), 7.90 (4H, d, J=8.7, 9.3Hz, 2", 6"-benH, 2, 6-benH), 7.72(2H, m, 4”-H, 4-H), 7.58(4H, t, m 3”, 5”-benH, 3, 5-H), 7.46(2H , d, J=8.5Hz, 2', 6'-ArH), 7.09 (2H, d, J=8.5Hz, 3', 5'-ArH), 6.77 (1H, d, 4 J=1.5Hz, 8-ArH), 6.39 (1H, d, 4 J=1.5Hz, 6-ArH); m / z (EI) 550.96 (M + +1, 100%).
Embodiment 3
[0040] Example 3 Compound 4', 5, the synthesis of 7-triphenylsulfonyloxy isoflavones
[0041] Genistein (0.4mmol, 0.108g) was dispersed in 10mL of dichloromethane, 0.01g of NaOH was added, and 1.44mmol (0.2541g) of benzenesulfonyl chloride was slowly added dropwise at -20°C under the protection of Ar. Incubate for 0.5-24 hours. Filter the mixture, spin the filtrate to dryness under reduced pressure, and purify by column chromatography (chloroform / acetone=50:1) to obtain a pure product.
[0042] 1 H NMR (400MHz, CDCl 3 ): δ7.98 (2H, d, J=7.6Hz, 2"", 6""-benH), 7.90 (4H, d, J=7.6Hz, 2", 6"-benH, 2, 6 -benH), 7.82 (1H, s, 2-H), 7.77 (1H, t, 4””-H), 7.72 (2H, t, 4”, 4-H), 7.58 (6H, m, 3 ", 3, 3"", 5", 5, 5""-benH), 7.39 (2H, d, J=8.5Hz, 2', 6'-ArH), 7.22 (1H, d, 4 J=1.88Hz, 8-ArH), 7.05 (2H, d, J=8.5Hz, 3', 5'-ArH), 6.81 (1H, d, 4 J=1.5Hz, 6-ArH); m / z (EI) 691.18 (M + +1, 100%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com