Method for synthesizing alpha-asarone
A technology of asarone and its mixture, which is applied in the field of synthesizing α-asarone, which can solve the problems of difficult removal, environmental pollution, separation and purification, etc., and achieve the effects of mild reaction, cost reduction and short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
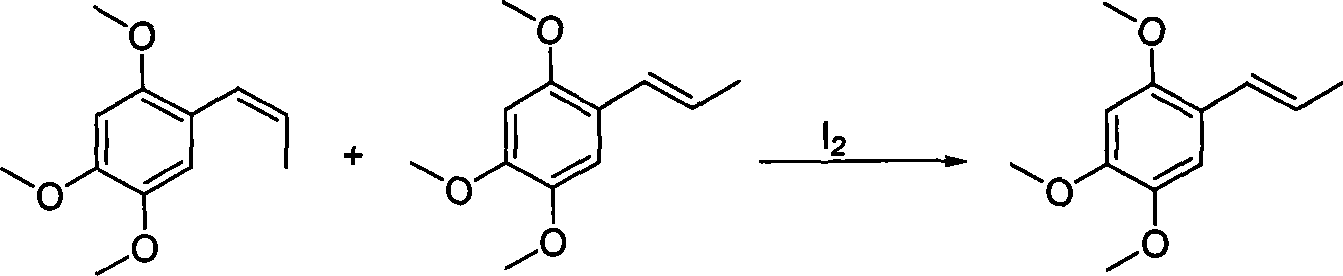
[0017] (1) Take 21.5g (0.11mmol) of 2,4,5-trimethoxybenzaldehyde, 61.25g (0.165mmol) triphenylethylphosphine bromide, 27.6g (0.20mmol) anhydrous K 2 CO 3 Powder, add 250ml dimethyl sulfoxide, stir and reflux for 24 hours. Stop the reaction, remove the solvent under reduced pressure, add 300ml petroleum ether and stir for 15 minutes, filter, wash the filtrate with 100ml (volume ratio 1: 1) of a mixed solution of ethanol and water, separate the oil layer, anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure to obtain 17.1 g of an oily mixture of α / β-asarone, with a yield of 60%. As determined by GC, α-asarone accounted for 43%, and β-asarone accounted for 57%.
[0018] (2) Get 5.0g (24.0mmol) of α / β-asarone mixture into a 100ml round bottom flask, add 25ml ethanol to dissolve; add catalyst I in the above solution 2 122.0mg (0.48mmol), stirred at room temperature for 12h, isomerization reached equilibrium, gas chromatography analysis trans / cis asa...
Embodiment 2
[0020] Get α / β-asarone mixture 5.0g (24mmol) in the 100ml round bottom flask, add 25ml ethyl acetate, add catalyst I 2 122.0mg (0.48mmol), stirred at room temperature for 30min, isomerization reached equilibrium, gas chromatography analysis showed trans / cis asarone=97.0 / 3.0, stop the reaction, the reaction solution was added successively 5% Na 2 S 2 o 3 solution and washed with water, anhydrous Na 2 SO 4 Drying, solvent removal, recrystallization with 7.5ml ethanol aqueous solution (V ethanol / V water=7: 3), obtains white crystal 3.65g, yield 73.0%, fusing point 60-61 ℃, GC analysis α-asarone content 98.0%.
Embodiment 3
[0022] Get α / β-asarone mixture 5.0g (24.0mmol) in the 100ml round bottom flask, add 25ml ethanol, add catalyst I 2 61.0mg (0.24mmol), stirred at room temperature for 24h, isomerization reached equilibrium, gas chromatography analysis trans / cis asarone = 95.0 / 5.0, stop the reaction, add 5% Na to the reaction solution 2 S 2 o 3 solution, after stirring for 30min, the solvent was removed, extracted three times with ethyl acetate, 30ml each time, washed with water, anhydrous Na 2 SO 4 Dry, extract solvent, recrystallize with 7.5ml ethanol aqueous solution (V ethanol / V water=7: 3), obtain white crystal 2.75g, yield 55% melting point 60-61 ℃, GC analysis α-asarone content is 98.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com