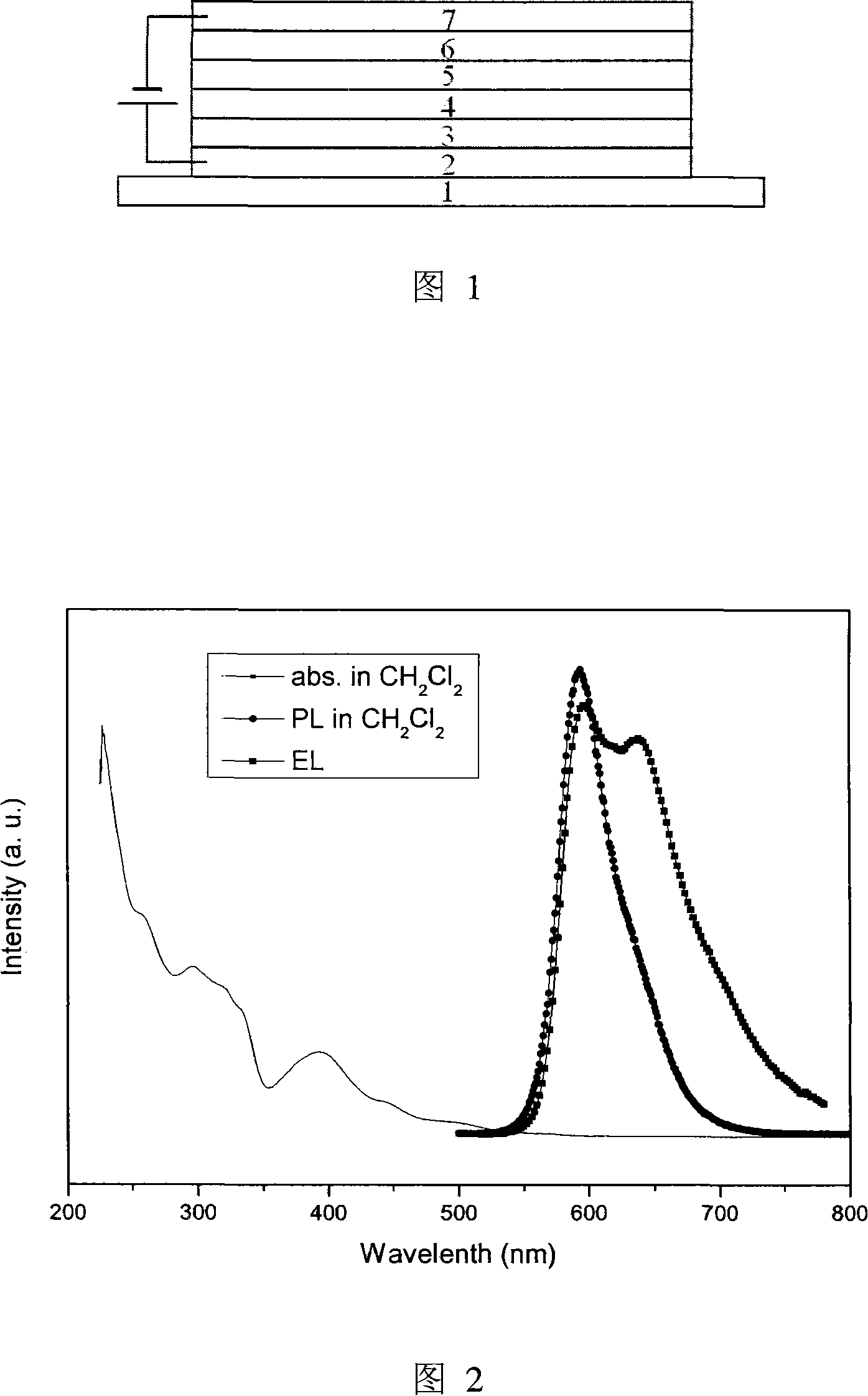
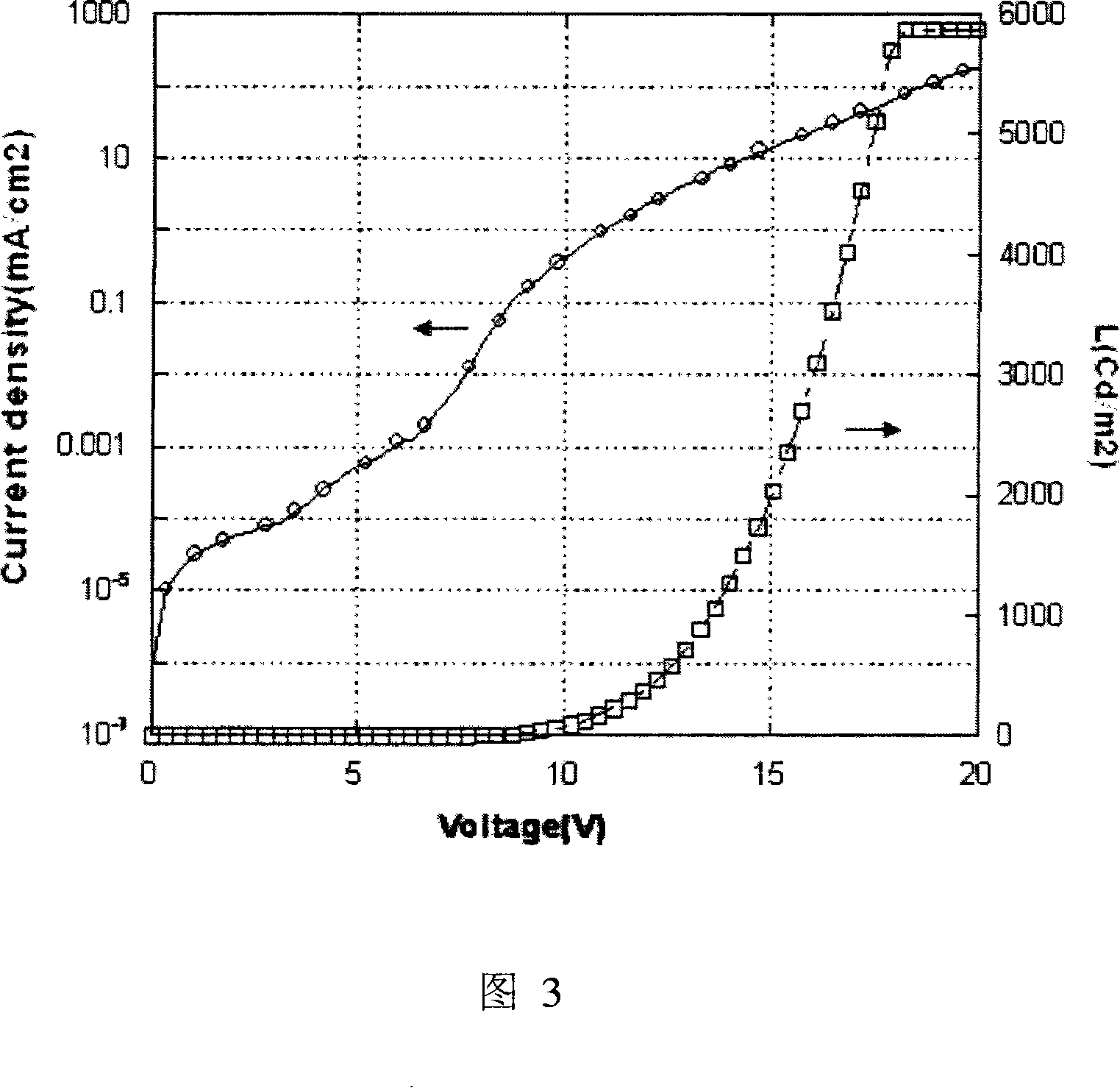
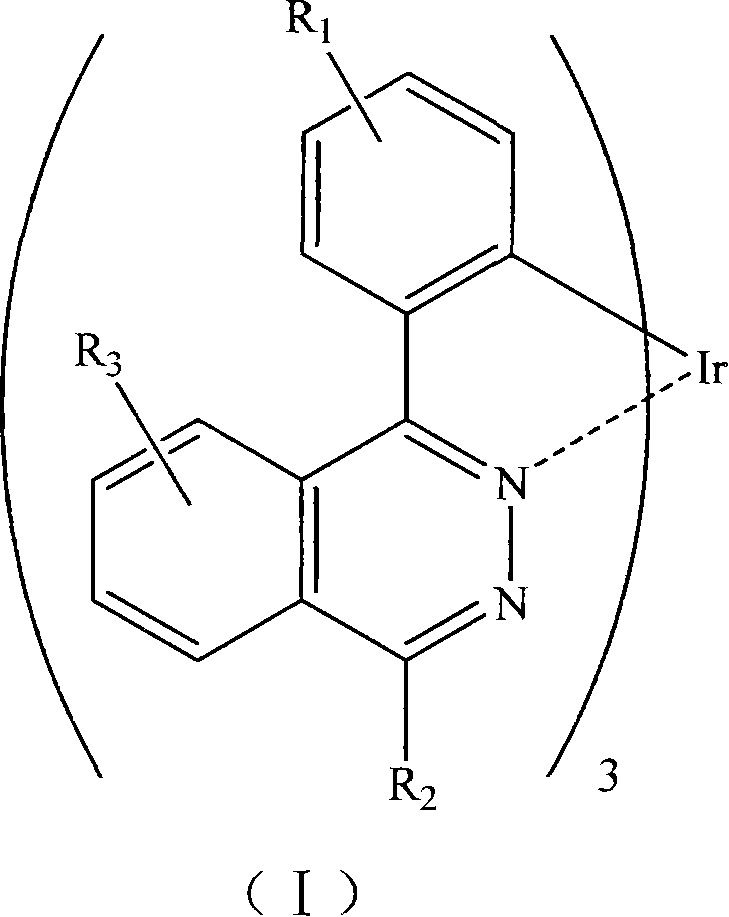
Phosphorescent iridium complex and organic electroluminescent device thereof
A phosphorescent iridium complex and organic layer technology is applied in the field of organic electroluminescence devices to achieve the effects of facilitating evaporation, reducing self-quenching phenomenon and reducing direct effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1. Ligand Synthesis
[0022] Add 12.2g (50mmol) of o-(p-chlorobenzoyl)benzoic acid and 2.5g (50mmol) hydrazine hydrate into a 250ml flask, add 150ml of ethanol, heat to reflux for 6h, and white crystals precipitate out, cool to room temperature, filter and weigh with ethanol Crystallize twice to give phthalazinone. Take 5g of phthalazinone, then put it into a 250ml flask, and add 10ml of POCl 3 and 150ml CHCl 3 , The reaction was refluxed for 6h, and then poured into water to obtain 4-chloro-1-chlorophenylphthalazine.
[0023] The above compound 5.7g, 2,6-dimethylphenol 2.7g, 5.4g K 2 CO 3 , 75ml of DMAc was added into a 150ml flask and reacted at 140°C for 2 hours, and the ligand was obtained after extraction and crystallization.
[0024] 2. Synthesis of complexes
[0025] Dissolve 1.4 g (4 mmol) of the ligand prepared above in a solution of ethylene glycol ether: water (volume ratio 12:4), and then add 0.35 g (1 mmol) of IrCl 3 ·3H 2 O, under nitrogen protecti...
Embodiment 2
[0032] 1. Ligand Synthesis
[0033] 2.63g (10mmol) of 4-chloro-1-chlorophenylphthalazine, 5g (30mmol) of carbazole, 13.8g (100mmol) K 2 CO3 , The catalyst is 1mol% Pd(OAc) 2 and 2mol%P(t-Bu) 3 75ml of xylene was added as a solvent into a 150ml flask and reacted at 120°C for 10 hours. After the reaction was completed, the solvent was vacuum-dried, passed through a silica gel chromatography column, and then recrystallized to obtain the ligand.
[0034] 2. Synthesis of complexes
[0035] Dissolve 3.57 g (4 mmol) of the above-prepared ligand in a solution of ethylene glycol ether: water (volume ratio 12:4), and then add 0.35 g (1 mmol) of IrCl 3 ·3H 2 O, under nitrogen protection, react at 80°C for 12h. The reaction solution was cooled to room temperature and then filtered. The filtrate was washed with water, ethanol, and n-hexane in sequence, and then separated by silica gel column chromatography using dichloromethane as the eluent to obtain a phosphorescent iridium complex ...
Embodiment 3
[0038] 1. Ligand Synthesis
[0039] Add 2.63g (10mmol) of 4-chloro-1-chlorophenylphthalein, 2.9g (50mmol) of potassium fluoride, and 100ml of sulfolane as a solvent into a 150ml flask and react at 150°C for 1 hour, then at 175°C for 12 hours. After the reaction was completed, the solvent was vacuum-dried, passed through a silica gel chromatography column, and then recrystallized to obtain the ligand.
[0040] 2. Synthesis of complexes
[0041] Dissolve 0.92 g (4 mmol) of the ligand prepared above in a solution of ethylene glycol ether: water (volume ratio 12:4), and then add 0.35 g (1 mmol) of IrCl 3 ·3H 2 O, under nitrogen protection, react at 80°C for 12h. The reaction solution was cooled to room temperature and then filtered. The filtrate was washed with water, ethanol, and n-hexane in sequence, and then separated by silica gel column chromatography using dichloromethane as the eluent to obtain a phosphorescent iridium complex with the structural formula (IV).
[0042] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Brightness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com