Method for synthesizing environment-friendly sucralose
A technology for sucralose and a synthesis method, which is applied to chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of low yield, unsuitability for industrial production, increased cost, and the like, and achieves improved chlorination selectivity. , Overcome the effect of many chlorinated derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
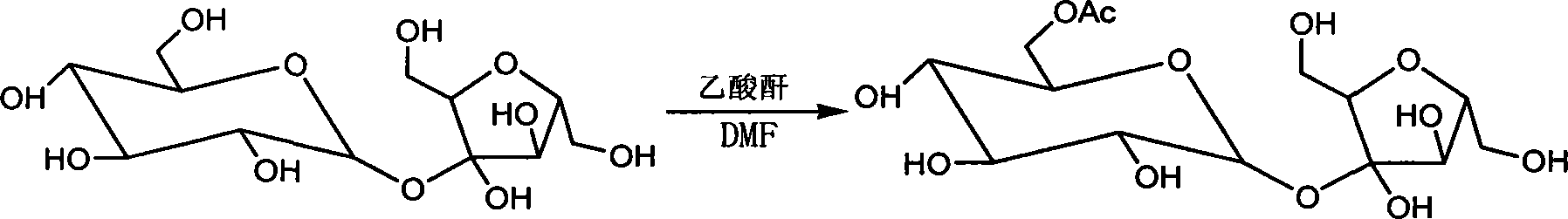
[0050] Example 1 Synthesis of sucrose-6-acetate
[0051] Under the protection of nitrogen, put 3.42Kg of sucrose and 10L of 1-methyl-3-methoxyimidazole hexafluorophosphate solution into a clean reaction kettle respectively, stir and dissolve, and detect that the moisture content is ≤0.2%, at 15-20°C Add a mixture of 1.34Kg acetic anhydride and 60g glacial acetic acid, react for 30min, cool down to 0°C, add 10L water, stir for 5min, add ethylenediamine to neutralize to pH=9, separate the water layer, wash the organic layer with 2L water, combine water layer, concentrate the aqueous layer under 30°C under a high vacuum of 5 to 10pa to obtain sucrose-6-acetate, the yield is 93.5%, and the content detected by HPLC is 93.5%, adding to sucrose-6-acetate 10L of 1-methyl-3-methoxyimidazolium tetrafluoroborate, stir well and set aside.
Embodiment 2
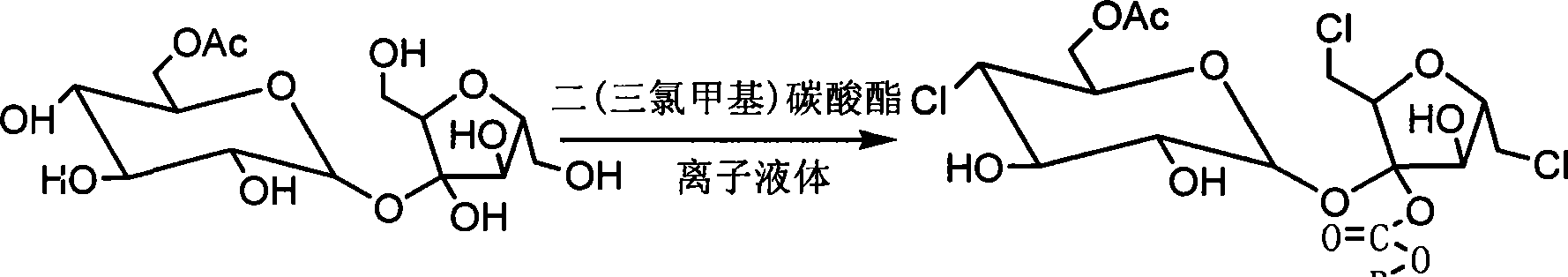
[0052] Example 2 Synthesis of sucralose-6-acetate
[0053] Under nitrogen protection, put 10L of dry 1-methyl-3-methoxyimidazole tetrafluoroborate solution into a clean reaction kettle, add 5.94Kg of bis(trichloromethyl)carbonate at room temperature, and stir to dissolve Then slowly put in the sucrose-6-acetate solution obtained in the previous step below 15°C. After feeding, slowly raise the temperature to 40°C and keep it for 2 hours. Lower the temperature, cool the reaction solution below 40°C, extract the sucralose-6-acetate intermediate compound three times with 15L ethyl acetate, combine the ethyl acetate layers, and neutralize to pH8 with 30% sodium hydroxide solution ~10, stirred for 1 hour, then neutralized with industrial hydrochloric acid to pH 6.5-7.5 to obtain sucralose-6-acetate, recovered ethyl acetate under reduced pressure to obtain concentrate sucralose-6-acetate syrup About 5.5Kg, then add 11L ethyl acetate to dissolve at 50°C, stir and crystallize at 0°C, ...
Embodiment 3
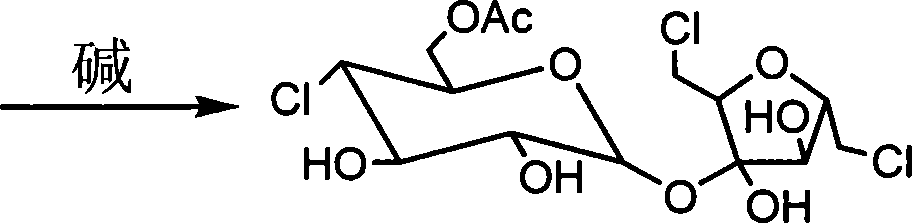
[0054] Example 3 Synthesis and crystallization of sucralose
[0055] Mix 1.0Kg of sucralose-6-acetate with 2.0Kg of absolute ethanol, stir to dissolve, then add 0.05Kg of triethylamine, heat to 40°C for 2 hours, then add 0.05Kg of activated carbon for decolorization for 15 minutes, filter the filtrate Heat to 80°C, after half of the ethanol is distilled off, add 1.0Kg butyl acetate and continue to distill until crystals are precipitated, stop heating after crystals are precipitated, cool to 38-40°C for 4 hours, then filter, and use a small amount of ethyl acetate The finished sucralose product is obtained by washing the filter cake and drying, with a yield of not less than 90% (calculated as sucralose-6-acetate), and a content of more than 99.5% as detected by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com