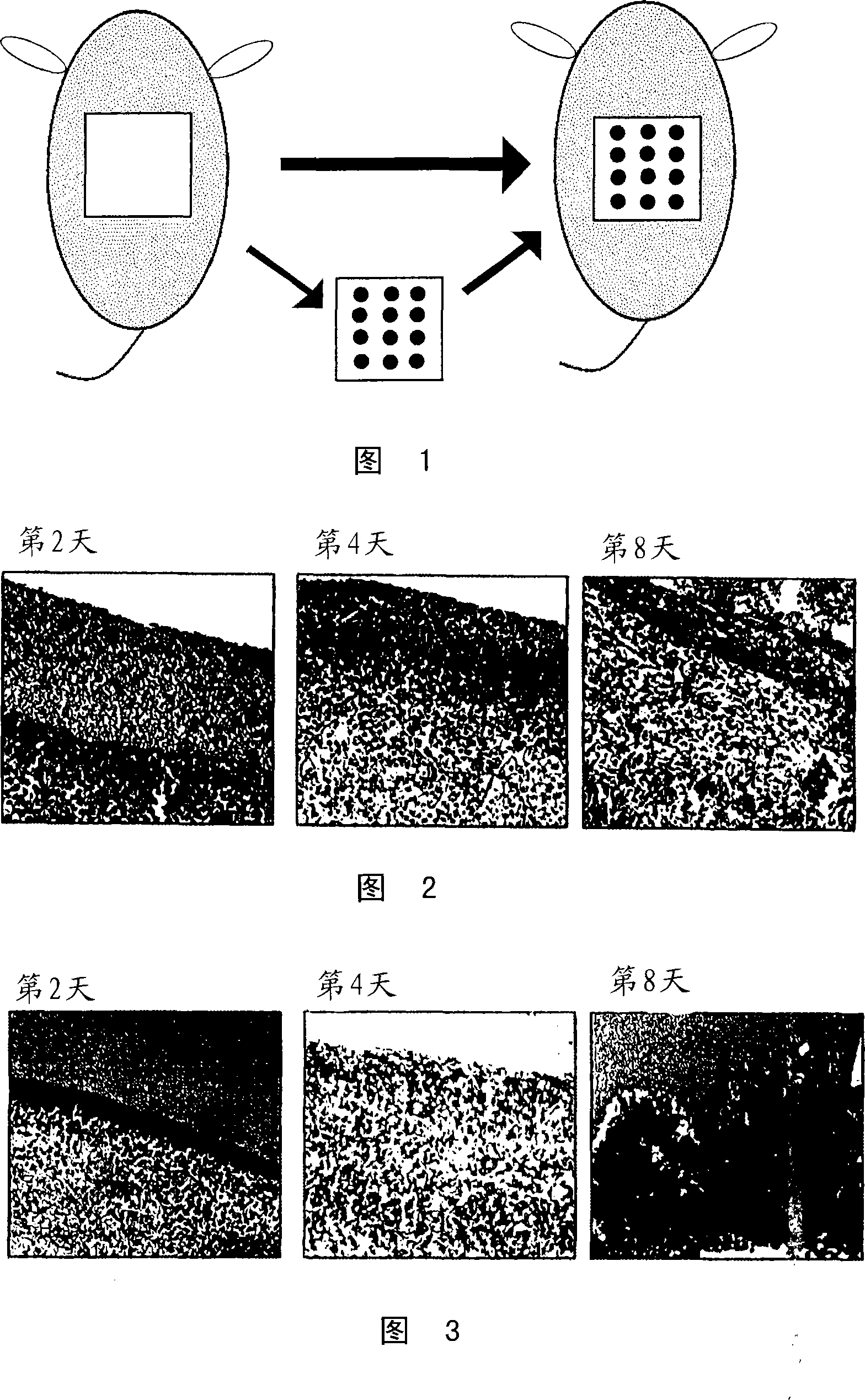
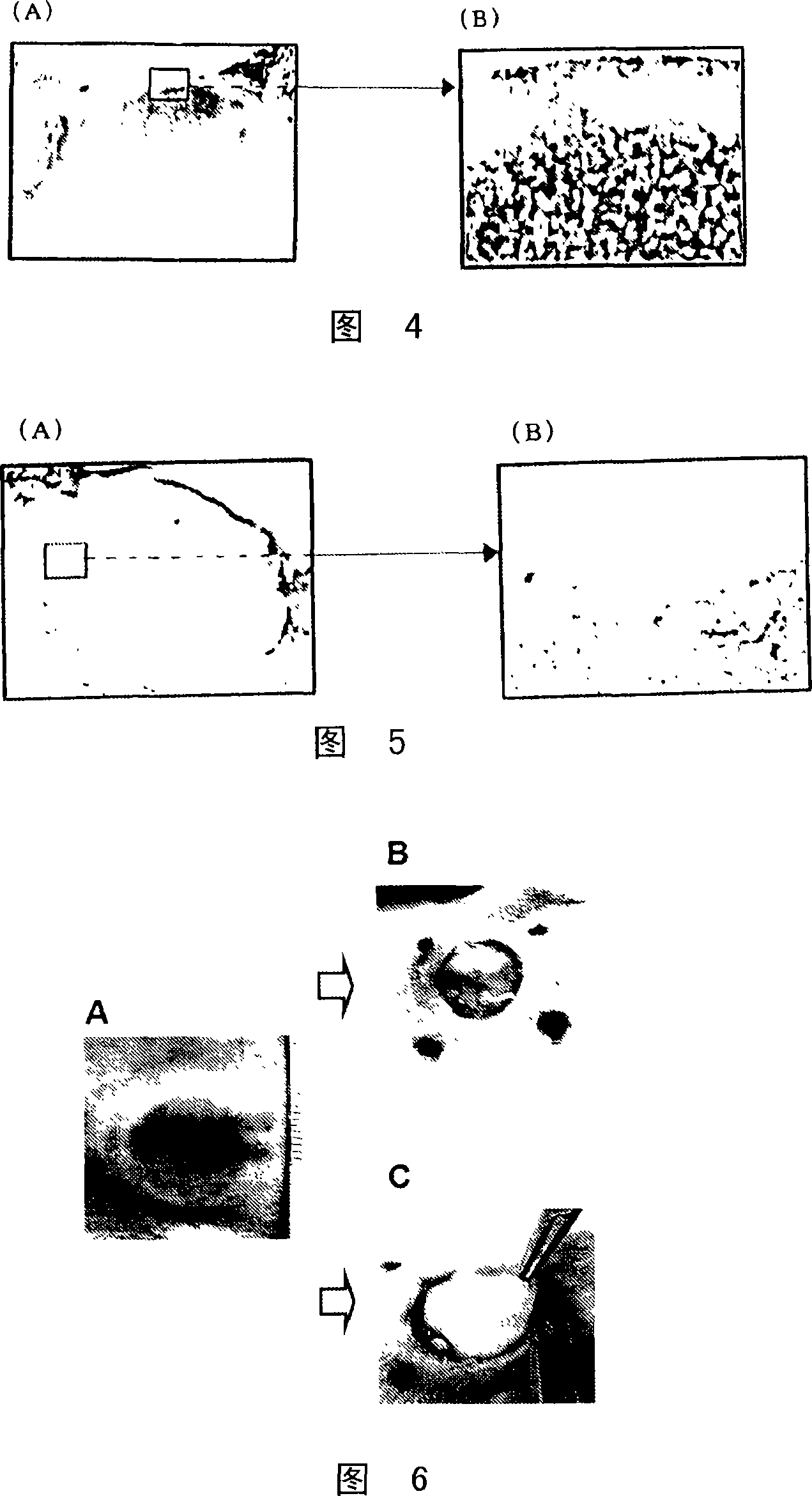
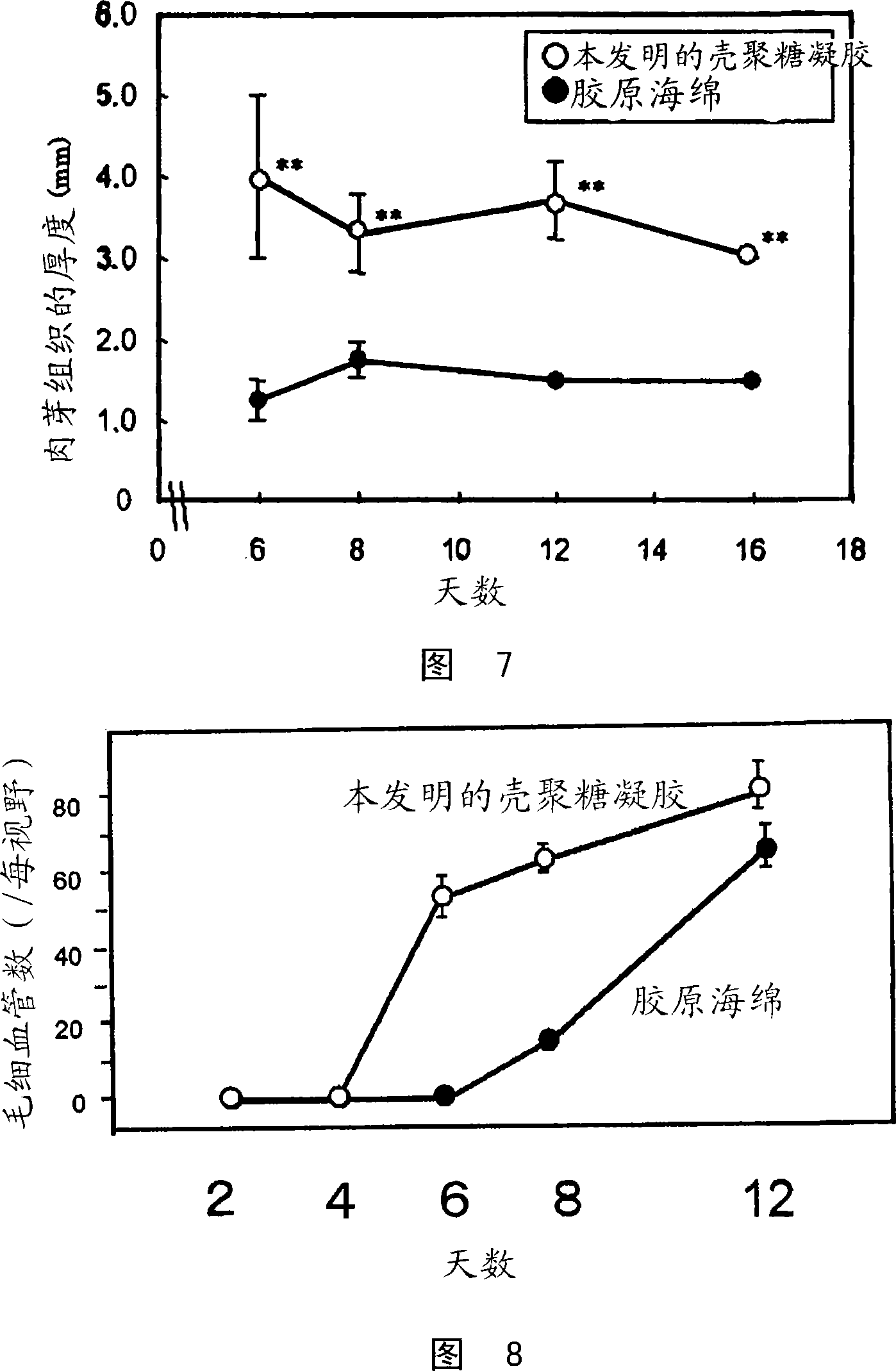
Medical composition for promotion of skin regeneration
A composition and technology of chitosan derivatives, which are applied in skin diseases, drug combinations, medical preparations with non-active ingredients, etc., can solve the problem of difficulty in ensuring epithelialization of wounds, postoperative bandage replacement and treatment pain or re-obstacles, It takes time and other issues to achieve the effect of improving wound healing activity, preventing infection of the affected area, and promoting epithelialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0092] Hereinafter, the present invention will be described in detail using specific examples, but the scope of the present invention is not limited to these specific examples.
Synthetic example 1
[0094] Synthesis of Photocrosslinkable Chitosan Derivatives (PRC)
[0095] According to the method described in WO00 / 27889, PRC having UV-reactive functional groups and sugar chains introduced into the chitosan backbone was synthesized. Specifically, an azide (p-azido group) was introduced into the amino group of shrimp-derived chitosan (manufactured by Yaizu Fisheries Industry Co., Ltd.) with a molecular weight of 800 to 1000 kDa and a degree of deacetylation of 85 through a condensation reaction. Benzoic Acid) and Lactose (Lactobionic Acid). By introducing lactose, it is soluble at pH in the neutral range, and the substitution degrees of p-azidobenzoic acid and lactobionic acid were confirmed to be about 2.5% and 5.0%, respectively.
[0096] In addition, the same derivatives can also be synthesized when chitosan raw materials derived from crab shells and cuttlefish cartilage are used.
Synthetic example 2
[0098] Synthesis of Light (Visible Light) Curable Chitosan Derivatives (VL-RC)
[0099] According to the method described in Journal of Polymer Science: Polymer Chemistry Edition, Vol.20, 1419-1432 (1982), methyl-4-[2-(4-formylphenyl)ethenyl] represented by the following formula was synthesized Pyridine mesylate (FPP).
[0100]
[0101] Specifically, a methanol (8.3 ml) solution of γ-picoline (3.07 g, 33 mmol) was added to dimethyl sulfate (4.16 g, 33 mmol) under ice-cooling. After the solution was left standing at room temperature for 1 hour, terephthalaldehyde (13.4 g, 100 mmol) was added and heated to dissolve. Then piperidine (0.47ml) was added and refluxed for 5 hours. The precipitate was filtered off while hot. The hot filtrate was combined with a mixed solvent of ethanol (50 ml) and acetone (16.7 ml), and left at room temperature overnight. The yellow precipitate was separated by filtration, washed with ethanol and acetone, and dried under reduced pressure to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com