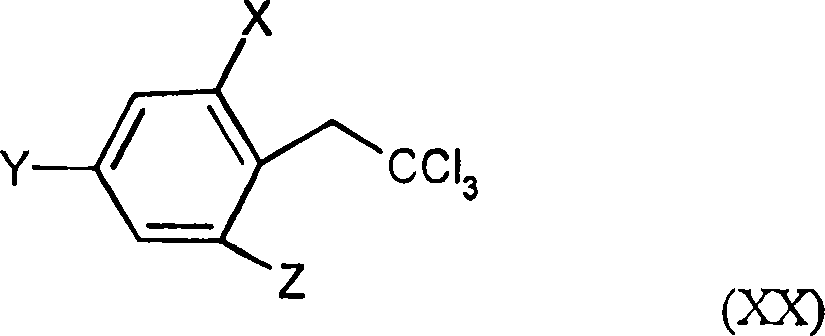
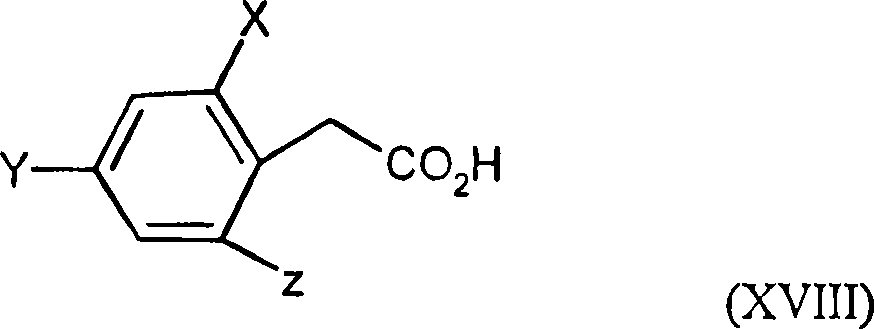
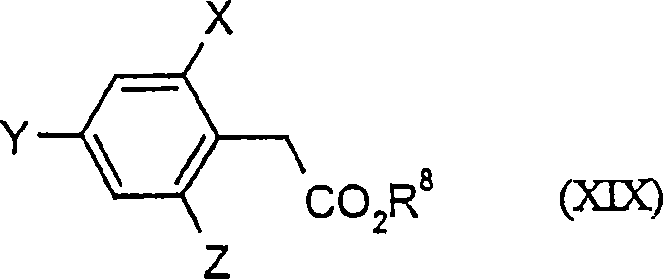2.4-dihalogen-6-(C2-C3-alkyl)-phenyl substituted tetramic acid derivatives
A brbrn-c3h7ch3, compound technology, applied in the field of tetramic acid derivatives substituted by 2,4-dihalogen-6-(C2-C3-alkyl)phenyl, can solve the problem that the compatibility is not always enough, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I-a-1
[0867]
[0868] At 0 to 20° C., a solution of 5.03 g of the compound of formula II-1 in 10 ml of anhydrous dimethylformamide was added to a solution of 2.92 g (0.023 mol) of potassium tert-butoxide in 8 ml of anhydrous dimethylformamide, and in The resulting mixture was stirred at 20°C.
[0869] The reaction solution was poured into 80 ml of ice water, and then the pH of the solution was adjusted to 1 with concentrated hydrochloric acid at 0-20° C., and the precipitate was filtered off with suction and dried. The product was then triturated with MTB ether / n-hexane.
[0870] Yield: 3.79 g (80% of theory), melting point: 245°C.
[0871] Similar to Example (I-a-1) and according to its generally described preparation method, the compound of the following formula (I-a) is obtained:
[0872]
[0873] Example
Numbering
X
Y
Z
D
A
B
Fp.℃
isomer
I-a-2 ...
Embodiment I-b-1
[0881]
[0882] 0.5 ml (3.6 mmol) of triethylamine was added to 1.3 g of the compound of Example I-a-1 in 30 ml of anhydrous ethyl acetate. A solution of 0.38 ml (0.0036 mmol) of isobutyryl chloride in 5 ml of anhydrous ethyl acetate was added dropwise under reflux.
[0883]The mixture was stirred at reflux. Reaction completion was checked by thin layer chromatography. The solvent was removed with a rotary evaporator, the residue was dissolved in dichloromethane and washed twice with 50 ml of 0.5N NaOH solution, dried and the solvent was evaporated off. The product was then recrystallized from MTB ether / n-hexane.
[0884] Yield: 0.81 g (55% of theory), melting point: 155°C.
[0885] Similar to Example (I-b-1) and according to its described general preparation method, the compound of the following formula (I-b) is obtained:
[0886]
[0887] Example
Numbering
X
Y
Z
D
A
B
R 1
...
Embodiment I-c-1
[0897]
[0898] At 0-10° C., a solution of 0.6 ml (0.006 mol) ethyl chloroformate in 50 ml of anhydrous dichloromethane was added dropwise to 50 ml of anhydrous dichloromethane and 0.84 ml ( 6mmol) in triethylamine. The mixture was stirred at room temperature until the reaction was complete (checked by TLC).
[0899] The solvent was then evaporated off, the residue was dissolved in dichloromethane and washed twice with 50 ml of 0.5N NaOH solution, dried, then the solvent was evaporated and the residue was recrystallized from MTB ether / n-hexane.
[0900] Yield: 2.2 g (79% of theory), melting point: 114°C.
[0901] Similar to Example (I-c-1) and according to its described general preparation method, the compound of the following formula (I-c) is obtained:
[0902]
[0903]
[0904] table (continued)
[0905] Example
Numbering
X
Y
Z
D
A
B
M
R 2
Fp.℃
isom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com