Method for treating advanced ovarian cancer with doxorubicin entrapped in liposomes
A technology of doxorubicin and liposomes, which can be used in liposome delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., and can solve problems such as toxicity and no overall survival rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
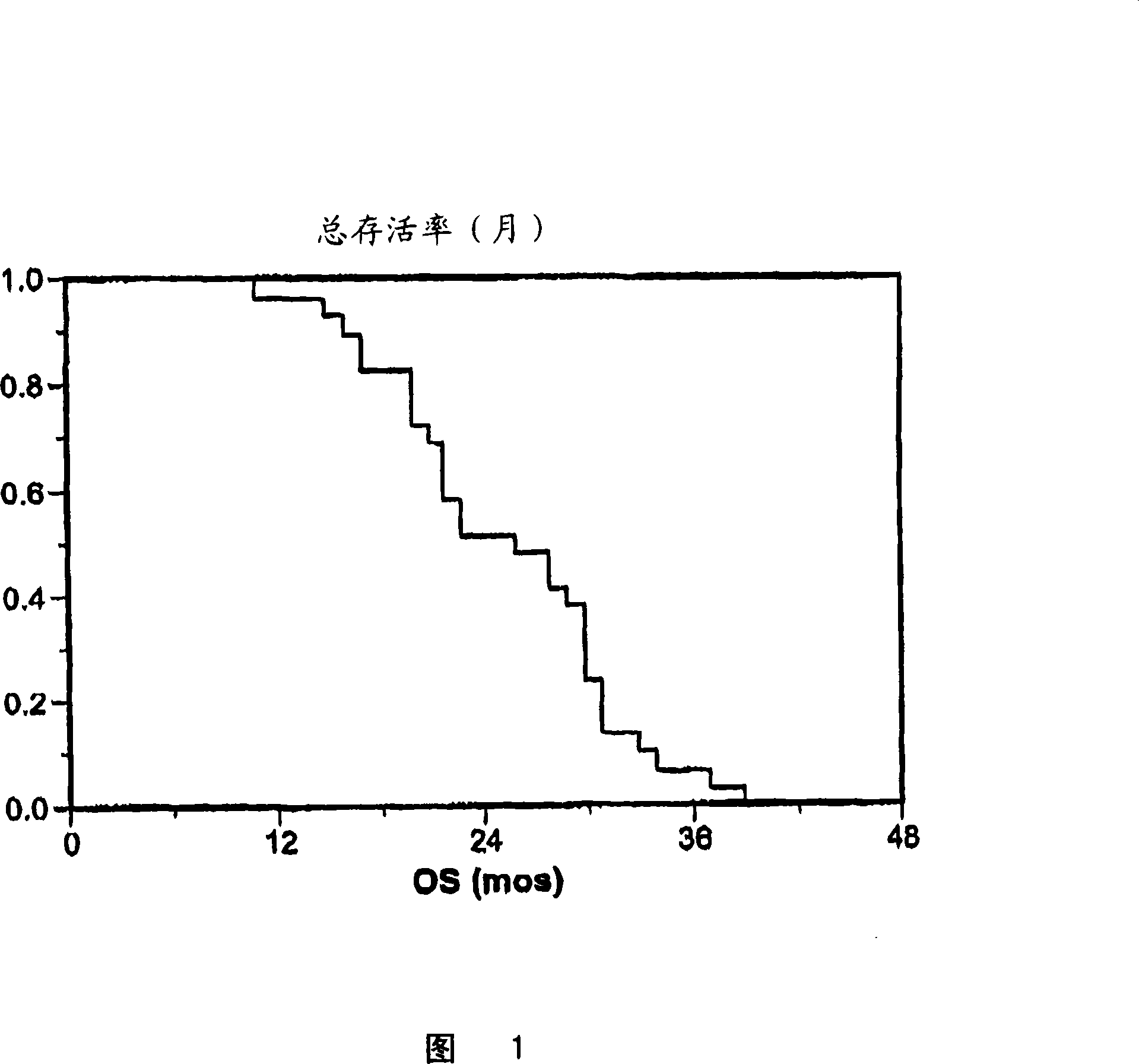
[0024] Treatment of patients with advanced ovarian cancer
[0025] Twenty-nine patients with suboptimally resected epithelial ovarian cancer who had been histologically determined to be FIGO stage IIIc or IV and who had achieved clinically definite completeness after six courses of platinum / paclitaxel-based chemotherapy were selected. answer. A clinically defined complete response was defined as no evidence of disease on physical examination and computed tomography testing, and CA-125 ≤ 35 units / mL. All patients had a physical status of 0-2 according to Zubrod's standard. The mean age of the patients was 57 (age range 46-79). At the time of initial cytoreductive surgery, 26 patients had FIGO stage IIIC tumors and 3 patients had stage IV tumors. All patients belonged to the case of suboptimal tumor resection. Eighteen patients had papillary serous histology, 6 patients had endometrioid histology, and 5 patients had mixed histology. The patient demographics are shown in Tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com