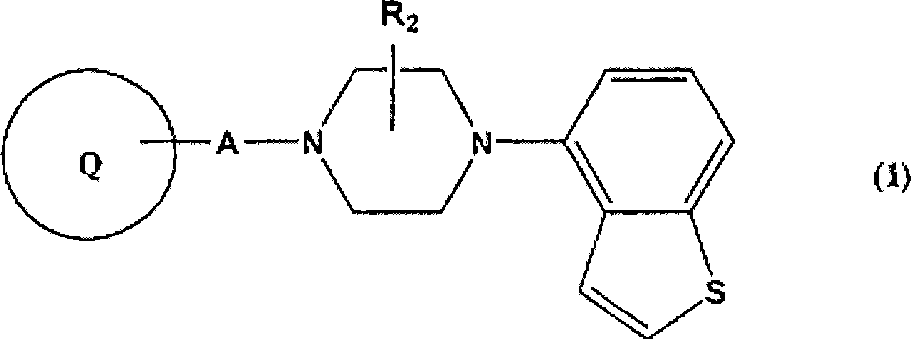
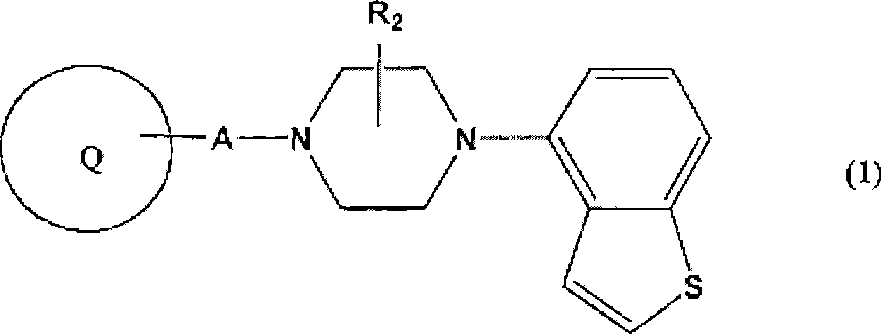
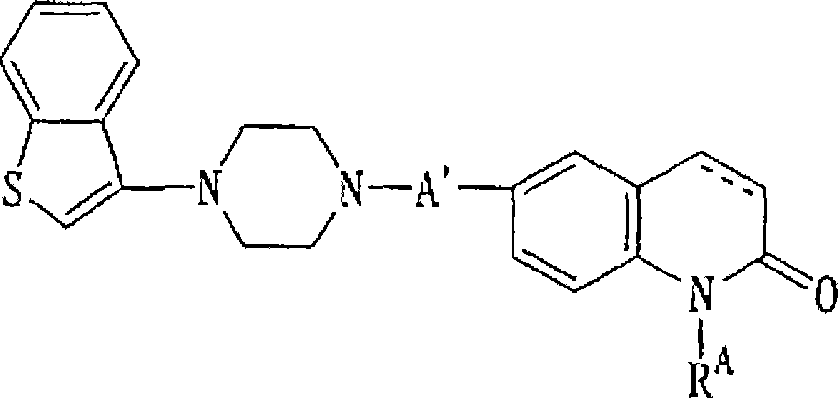
Piperazine-substituted benzothiophenes for treatment of mental disorders
A technology of substituents and representatives, applied in the field of new heterocyclic compounds, can solve problems such as not explicitly disclosing compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0358] Preparation of 7-[4-(4-benzo[b]thiophen-4-yl-piperazin-1-yl)butoxy]-1H-quinolin-2-one
[0359] 9.0g 7-(4-chlorobutoxy)-1H-quinolin-2-one, 10g 1-benzo[b]thiophen-4-yl-piperazine hydrochloride, 14g potassium carbonate, 6g sodium iodide and 90 ml of dimethylformamide were stirred at 80°C for 2 hours. Water was added to the reaction solution, and the precipitated crystals were separated by filtration. The crystals were dissolved in a mixed solvent of dichloromethane and methanol, dried over magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel column chromatography (dichloromethane:methanol=100:3). Recrystallization in ethanol prepared 13.6 g of 7-[4-(4-benzo[b]thiophen-4-yl-piperazin-1-yl)butoxy]-1H-quinoline-2 in the form of a white powder -ketone.
[0360] The melting point is 183.5-184.5°C
[0361] 1 H-NMR (DMSO-d 6 )δppm:
[0362] 1.6-1.75(2H, m), 1.75-1.9(2H, m), 2.44(2H, t, J=7Hz), 2.5-2.8(4H, m), 2.9...
Embodiment 2
[0364] Preparation of 3-[2-(4-benzo[b]thiophen-4-yl-piperazin-1-yl)ethoxy]-1H-quinolin-2-one
[0365] Using a method similar to that in Example 1, 3-[2-(4-benzo[b]thiophen-4-yl- piperazin-1-yl)ethoxy]-1H-quinolin-2-one.
[0366] white powder (chloroform)
[0367] The melting point is 201.9-204.5°C
[0368] 1 H-NMR (CDCl 3 )δppm:
[0369] 2.90-2.95(4H, m), 3.10(2H, t, J=5.9Hz), 3.23-3.27(4H, m), 4.30(2H, t, J=5.9Hz), 6.9O(1H, d, J =7.7Hz), 7.08(1H, s), 7.15-7.32(2H, m), 7.37-7.41(4H, m), 7.47-7.49(1H, m), 7.55(1H, d, J=8.1Hz) , 11.33(1H,br).
Embodiment 3
[0371] Preparation of 7-[3-(4-benzo[b]thiophen-4-yl-piperazin-1-yl)propoxy]-4-methyl-1H-quinolin-2-one
[0372] Using a method similar to that in Example 1, 7-[3-(4-benzo[b]thiophene was prepared from 7-(3-chloropropoxy)-4-methyl-1H-quinolin-2-one -4-yl-piperazin-1-yl)propoxy]-4-methyl-1H-quinolin-2-one.
[0373] Light brown powder (ethyl acetate)
[0374] The melting point is 202-208°C
[0375] 1 H-NMR (DMSO-d 6 )δppm:
[0376] 1.95-2.0(2H, m), 2.37(3H, s), 2.55(2H, t, J=7Hz), 2.6-2.7(4H, m), 3.05-3.2(4H, m), 4.09(2H, t , J=6.5Hz), 6.21(1H, bs), 6.8-6.85(2H, m), 6.90(1H, d, J=7.5Hz), 7.28(1H, dd, J=8Hz, 8Hz), 7.41( 1H, d, J=5.5Hz), 7.6-7.7(2H, m), 7.69(1H, d, J=5.5Hz), 11.41(1H, bs).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com