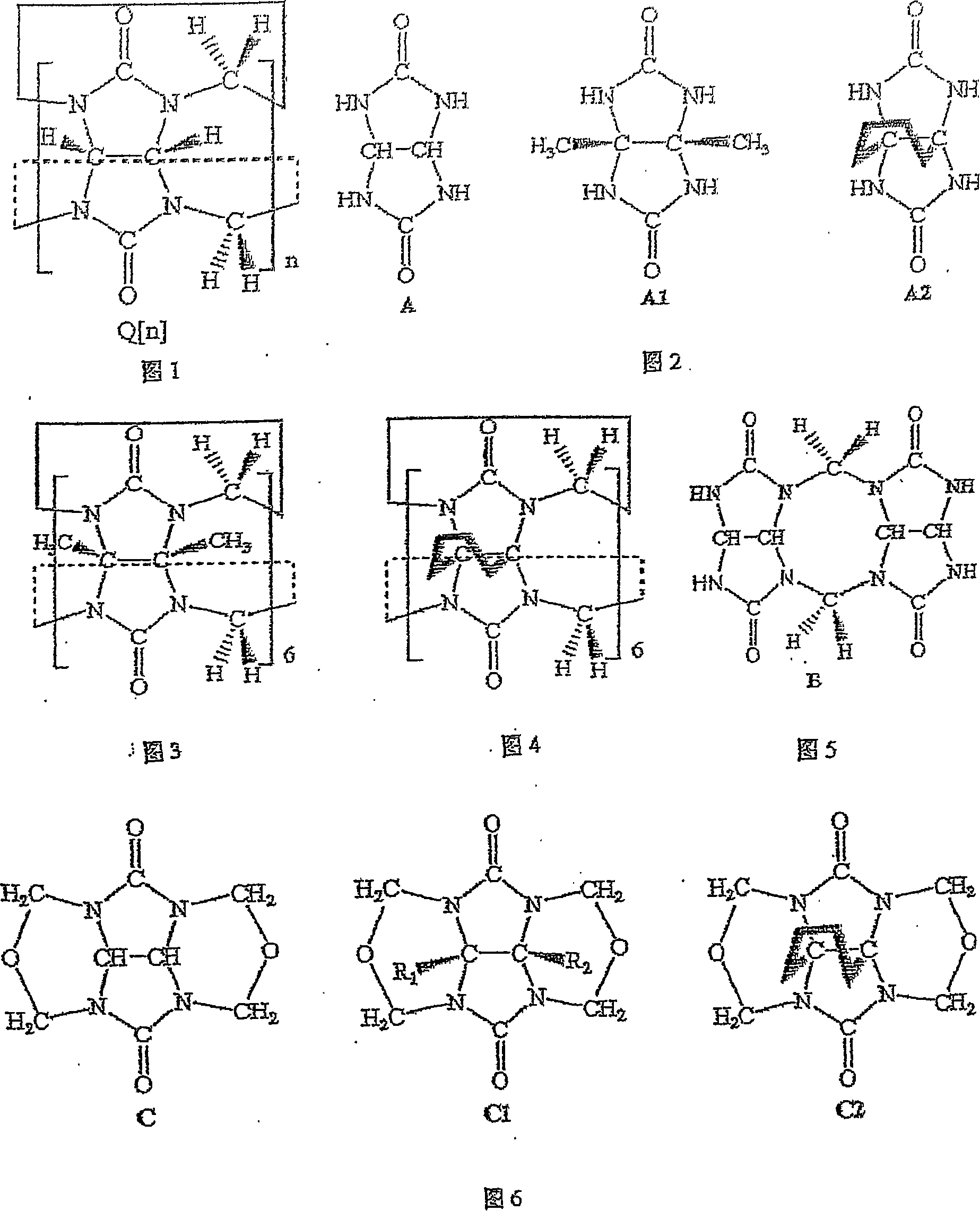
Melon ring and method of synthesizing substituted melon ring
A synthetic method, the technology of cucurbit rings, applied in the field of compound synthesis, can solve problems such as effective control of the number and position of substituents, difficulty in obtaining products containing only part of substituents, difficult substitution of cucurbit rings, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: In a 250mL round bottom flask filled with concentrated hydrochloric acid (40mL), add dimethyl-substituted epoxy glycoluril (4.0g, 20mmol), glycoluril dimer (8.0g, 26mmol), diglycoside The ratio of the amount of the polymer to the substance of the dimethyl-substituted epoxyglycoside is 1.3:1, and potassium chloride (2.0 g, 26 mmol) is heated under reflux under stirring for 2 hours. After the obtained dark red solution was cooled to room temperature, light red crystals (mainly composed of potassium chloride) appeared, and the crystals were filtered. After the filtrate was concentrated on a rotary evaporator to remove hydrochloric acid, 10 g of mixed cucurbit rings was obtained with a yield of 85%. Add 500mL of water to the crude product to dissolve the soluble melon ring in the mixture. The eluate was concentrated and recrystallized with distilled water to obtain 6.0 g of hexamethylpentaquinone (HMeQ[5]), with a yield of 49%. The precipitate was recrystallize...
Embodiment 2
[0035]Embodiment 2: take by weighing 2g dimethyl epoxidized glycoluril and 3.62g glycoluril dimer (its mol ratio is 1: 1), and lithium chloride (as template reagent) 0.5g, join in 19ml 36% In the hydrochloric acid solution, stir at room temperature until the reactants are completely dissolved (about 1 hour), the reaction solution turns dark red, and then heated to 90-100 ° C for 2 hours, after the obtained dark red solution is cooled to room temperature, crystals appear 1:1), and lithium chloride (as a template reagent) 0.5g, was added to 19ml of 36% hydrochloric acid solution, stirred at room temperature until the reactants were completely dissolved (about 1 hour), the reaction solution turned dark red, and then heated React at 90-100°C for 2 hours, and after cooling the resulting dark red solution to room temperature, crystals (mainly composed of lithium chloride) appear. The crystal filtration reaction solution was evaporated to dryness with a rotary evaporator to obtain 4....
Embodiment 3
[0036] Embodiment 3: in the hydrochloric acid solution of 60ml 36%, glycoside uride dimer 12.0g, lithium chloride (as template reagent) 7.5g, paraformaldehyde 1.3g, the substance of glycoside uride dimer and paraformaldehyde The amount ratio is 1:1.11. After dissolved under stirring, heat to reflux under stirring at 100°C for 2 hours, cool the obtained solution to room temperature, and filter. After the filtrate was concentrated on a rotary evaporator to remove most of the hydrochloric acid, the clear solution obtained by suction filtration and the filtrate standing still crystallized to obtain 11 g of crude melon ring product with a yield of 88%. The crude product of mixed cucurbit rings is dissolved in as little as possible 2mol / L HCl eluent, and is separated by column chromatography (chromatographic column: chromatography silica gel 500g, column length L=30cm, column diameter R=5cm), to obtain Six-membered melon ring (Q[6]) 9.0g and eight-membered melon ring (Q[8]) 1.1g. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com