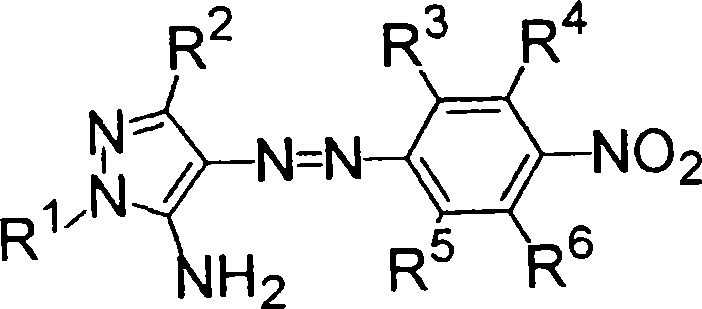
Azo dye, heat-sensitive transfer recording ink sheet, heat-sensitive transfer recording method, color toner, inkjet ink and color filter
A technology of azo dyes and thermal transfer printing, which is applied in the direction of azo dyes, monoazo dyes, optical filters, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
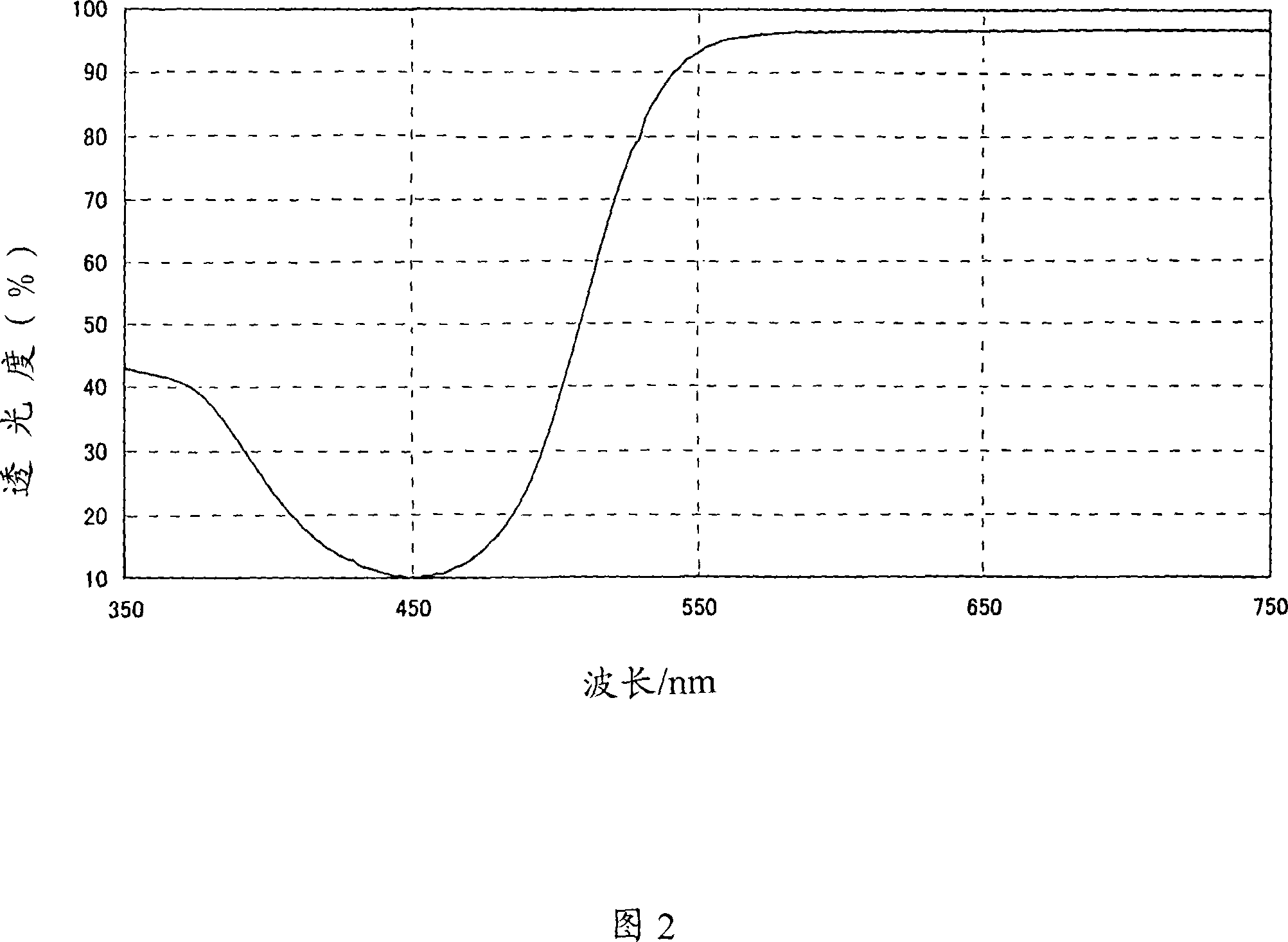
Image
Examples
Embodiment 1
[0127] Synthesis Example 1 (Preparation Example 1 Compound (1))
[0128]
[0129] A mixture of 41.4 g of 4-nitroaniline (0.3 mol), 84 ml of concentrated hydrochloric acid, 60 ml of acetic acid and 84 ml of propionic acid was stirred at an inner temperature of 0-5°C. To this mixture was added dropwise 20.7 g (0.3 mol) of sodium nitrite per 45 ml of water at an internal temperature of 5°C or lower. Stirring was then continued for 30 min at an internal temperature of 0-5°C. At an internal temperature of 10°C or lower, the obtained azide salt solution was added dropwise to 600 ml of in acetonitrile. After the dropwise addition of the azide salt solution, the resulting reaction solution was stirred for 2 h and then poured into 1000 ml of water. The precipitated azo dye was then isolated by filtration. The obtained crystals were recrystallized from 1650 ml of methanol to obtain Exemplified Compound (1). Yield: 72 g (80%), melting point: 199-201°C. The λmax (ethyl acetate so...
Embodiment 2
[0131] Synthetic Example 2 (preparation example compound (2))
[0132]
[0133]
[0134] 150 ml of an acetonitrile solution containing 11.1 g (0.08 mol) of 5-tert-butyl-2H-pyrazol-3-ylamine was cooled to an internal temperature of 0-5°C. 19.0 g (0.08 mol) of p-nitrophenylfluoroborate diazonium salt was added in batches to this solution. The reaction mixture was then stirred at an internal temperature of 0-5° C. for 30 min, and at room temperature for 1 h. Then pour into 500ml of water. After extraction with 200 ml of ethyl acetate, the ethyl acetate phase was washed twice with saturated aqueous sodium bicarbonate solution, and then washed twice with saturated brine. Ethyl acetate containing the dye was heated to an internal temperature of 55°C, and 600 ml of hexane was added. Recrystallization affords intermediate (2a). Yield: 15.7 g (68%).
[0135]
[0136]A mixture of 2.88 g (0.01 mol) of intermediate (2a), 1.38 g (0.01 mol) of sodium carbonate and 15 ml of dimet...
Embodiment 3~11
[0138] Exemplary compounds (3)-(11) were synthesized according to the methods of Synthesis Examples 1 and 2. In the exemplified compounds (3)~(11) thus obtained in ethyl acetate solution (density: 1×10 -6 mol / L, optical channel length: 10 mm), the maximum absorption wavelength of each absorption spectrum and the melting point of each such compound are expressed together with the exemplary compounds (1) and (2) obtained in Synthesis Examples 1 and 2 as follows.
[0139] Table 1
[0140] dye
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com