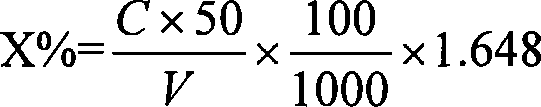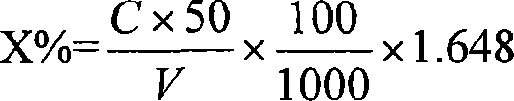Method for determining sodium chloride content in phosphate using spectrophotometry
A technology of spectrophotometry and sodium chloride, which is applied in measuring devices, analyzing materials, and analyzing materials through optical means. It can solve problems such as excessive chloride ions in boiler water, deterioration of soda quality, and inability to effectively control them. The method is simple , The effect of less instrument maintenance workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] Below in conjunction with example the present invention will be further described.
[0024] The present invention is aimed at the chloride ion in phosphate, in the solution containing chloride ion, chloride ion reacts with mercury thiocyanate, generates mercury chloride and releases SCN - ; In perchloric acid medium, Fe 3+ with SCN - Form a stable orange-red complex, the absorbance of this complex and the content of chloride ion are between 0~1.0CI - In the range of mg / L, there is a linear relationship. Draw (A-A 0 )-C straight line or the absorbance of the solution (A-A 0 ) and the concentration C to perform regression calculation, the regression equation is obtained: A-A 0 =a+bC.
[0025] First, take high-purity water, ferric nitrate-perchloric acid solution, mercury thiocyanate-methanol solution and chloride ion standard solution for use.
[0026] The high-purity water has an electrical conductivity less than 0.1 μs / cm; the ferric nitrate-perchloric acid solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com