7-azaindirubin and 7-azaisoindigo derivative, its production and pharmaceutical use
A derivative, indirubin technology, is applied in the field of preparation of 7-azadirubin and 7-azaisoindigo derivatives and their pharmaceutical uses, which can solve problems such as poor fat solubility, improve physical and chemical properties, improve Bioavailability, the effect of enhancing cell permeability
Active Publication Date: 2007-11-21
JC (WUXI) CO INC
View PDF1 Cites 21 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0006] In summary, indirubin-like bisindole compounds are an important class of CDKs inhibitors with low toxicity and side effects, but their fat-solubility and water-solubility are poor, which affects their clinical application. In recent years, foreign Many pharmaceutical research institutes and pharmaceutical companies have carried out extensive structural modifications to indirubin compounds (WO: 99 / 62503, 1999-12-9; WO: 01 / 37819 A2, 2001-8-31; WO: 00 / 61555, 2000-10-19; US pat: 2002 / 0132792 A1, 2002-9-19), the properties such as the dissolution of these compounds are improved to varying degrees, in order to further improve the antitumor properties of indirubin bisindole compounds effect, it is necessary to make major changes to the structure of such compounds
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
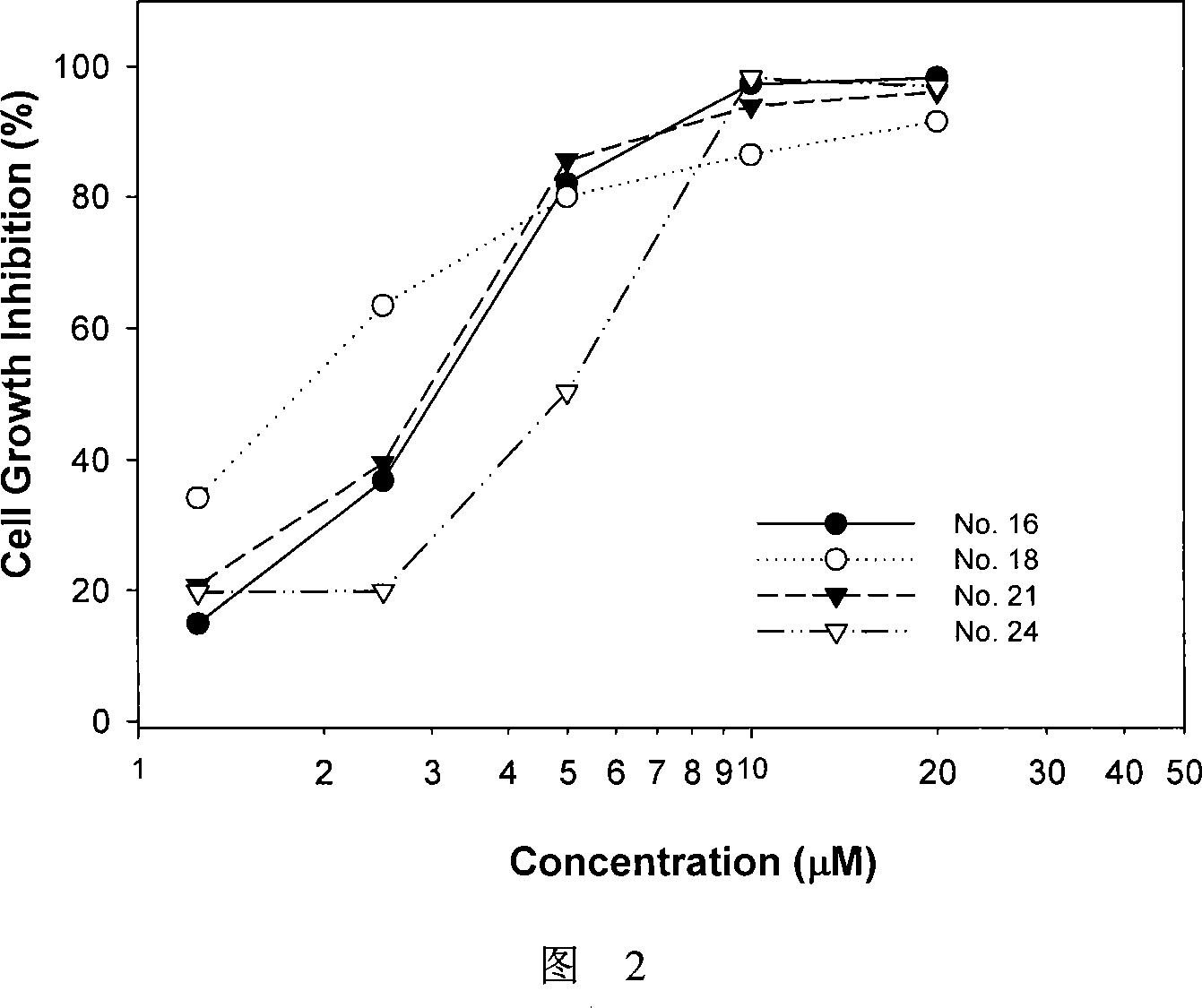
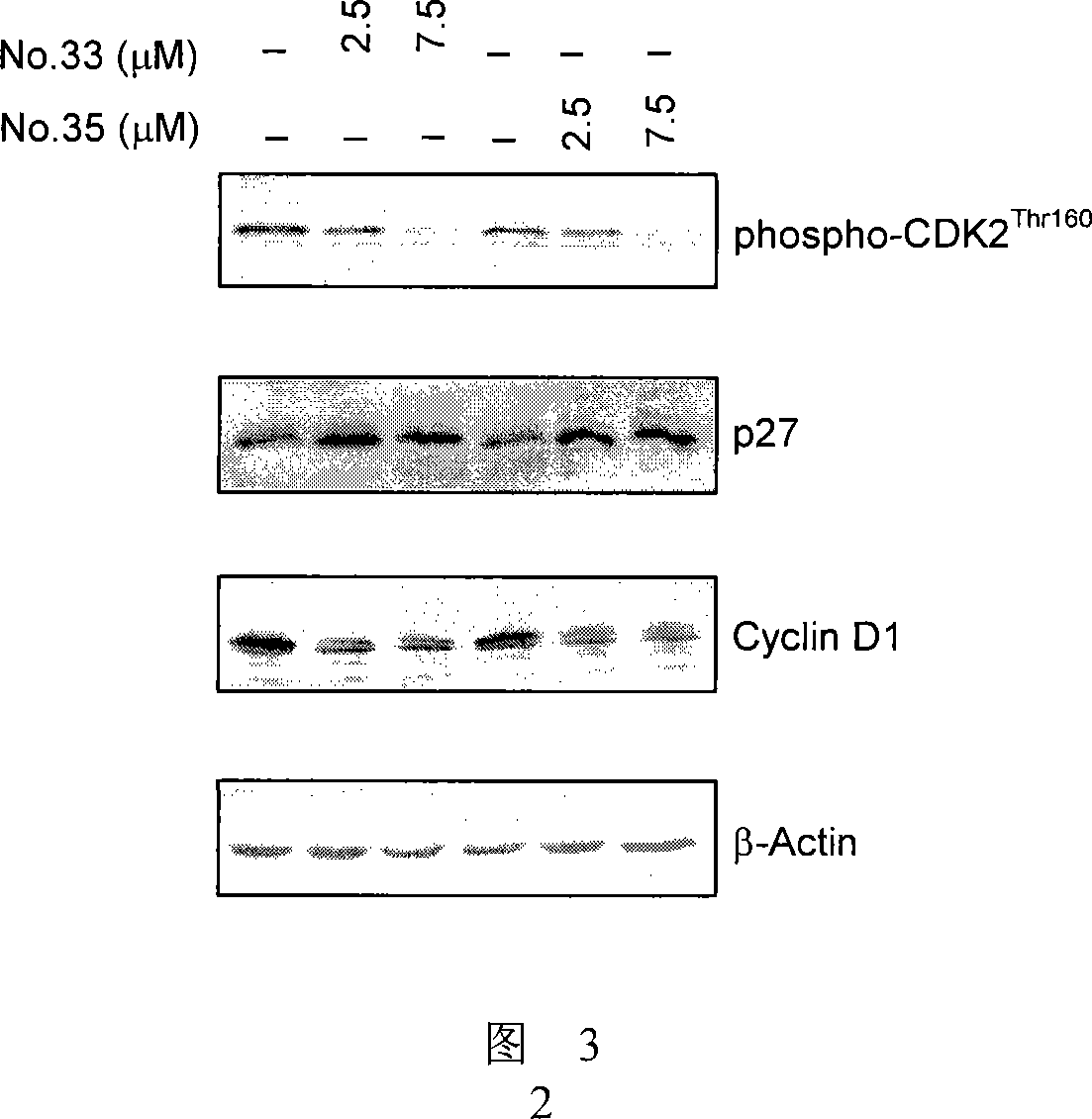
Effect test
Embodiment
[0063] 1. Instruments and reagents
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Login to View More
Abstract
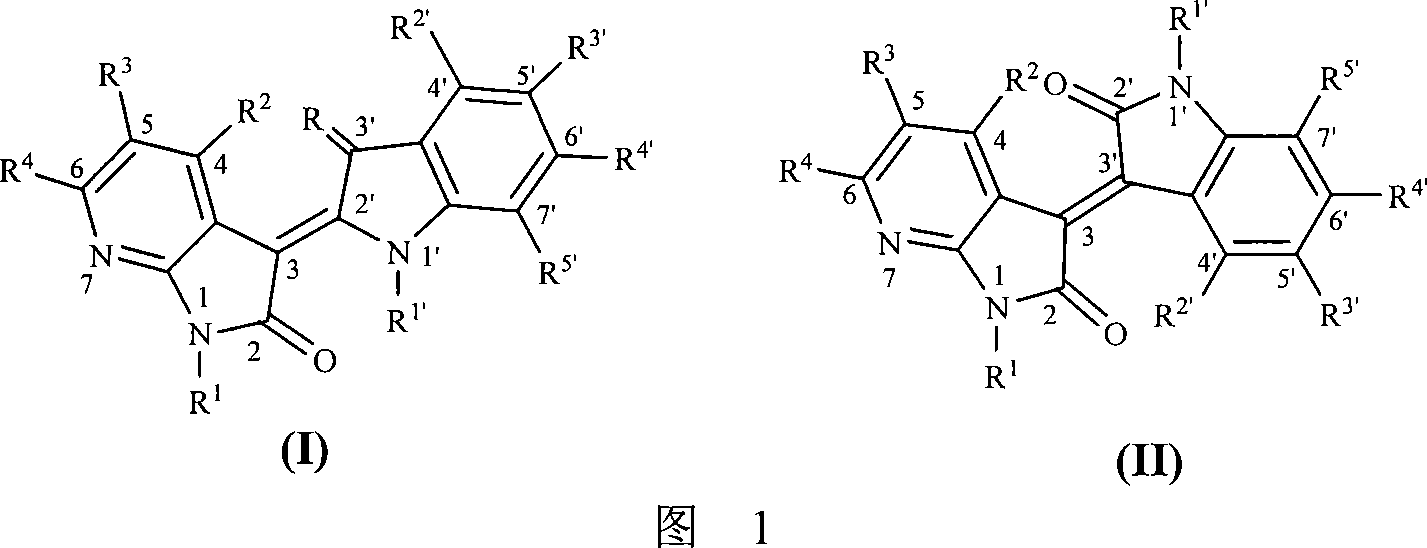
A method for designing and synthesizing 7-azaindirubin and 7-azaisoindigo derivative and its medicinal use are disclosed. It changes indirubin and isoindigo nuclear-parent molecule atomic composition to form into compound. It also changes original molecule electric property. The process is carried out by coupling at different position by 7-azaindole and indole bi-molecule, inhibiting CDKs and CDKIs growth and breed and inducing CDKIs biological function. It can be used to treat malignant tumor, HIV and nervous system diseases.
Description
technical field [0001] The present invention relates to a method for designing and synthesizing a class of novel structure 7-azaidirubin and 7-azaiisoindigo derivatives and their pharmaceutical application. The common feature of these molecules is that they are all formed by coupling 7-azaindole and indole bimolecules at different positions, and inhibit cell growth and proliferation through various mechanisms, including inhibition of cyclin-dependent kinases ( Cyclin-dependent kinases, referred to as cell cycle kinases, abbreviated as CDKs), inducing endogenous cell cycle kinase inhibitors (CDKIs) and other biological functions, and then have the ability to treat various diseases caused by cell growth disorders, including malignant tumors, Neurological diseases such as viral skin diseases, HIV, neurodegeneration and disorders, etc. Background technique [0002] The cyclin-dependent kinases (cyclin-dependent kinases, abbreviated as cell cycle kinases, abbreviated as CDKs) fa...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D471/04A61K31/437A61P35/00
CPCC07D471/04A61P17/00A61P17/06A61P25/00A61P31/18A61P35/00A61P37/00A61P43/00A61P5/50A61P3/10
Inventor 姚其正王朝晖程景才华维一
Owner JC (WUXI) CO INC
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com