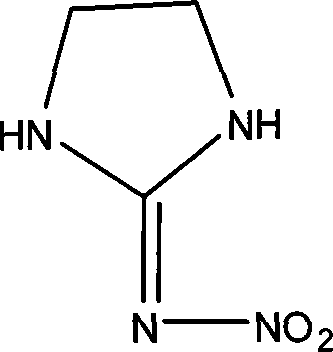
Prepn process of 2-nitro imido imidazolyl alkane
A technology of nitroiminoimidazolidine and nitroguanidine, which is applied in the field of preparation of 2-nitroiminoimidazolidine, can solve the problem of low yield of 2-nitroiminoimidazolidine, low yield, potassium hydroxide Problems such as high cost of raw materials, to achieve the effect of good product quality, high reaction yield, and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
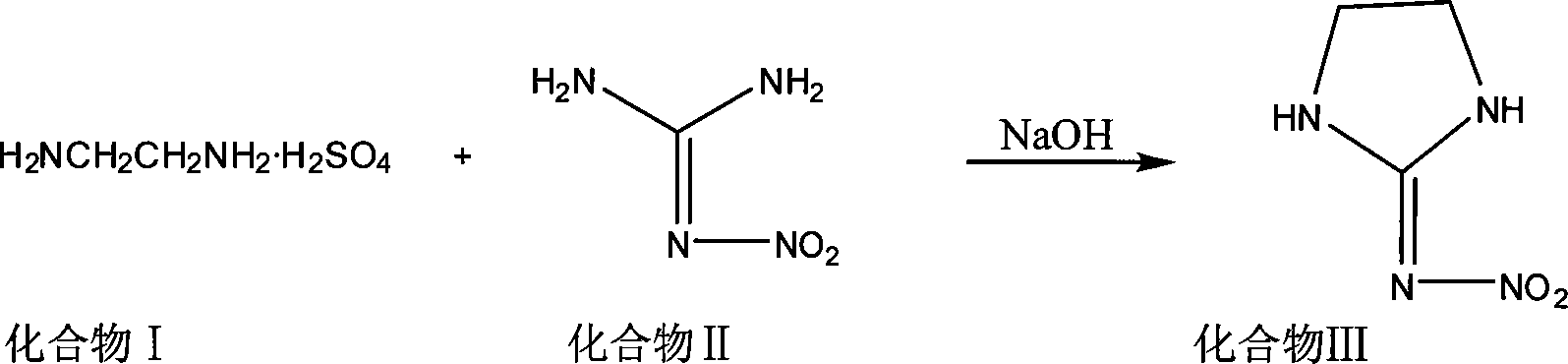
[0018] Pump 150Kg ethylenediamine and 300Kg water into a 1000L reactor, add concentrated sulfuric acid dropwise to neutrality under freezing, put in 260Kg nitroguanidine, stir evenly, add 30% liquid caustic soda dropwise until the pH is 9, heat up to 60°C, and react After 6 hours, cool to room temperature, shake and filter to obtain a wet product, and dry to obtain a 254Kg finished product (above the content of 99%).
Embodiment 2
[0020] Pump 150Kg ethylenediamine and 300Kg water into a 1000L reactor, add concentrated sulfuric acid dropwise to neutrality under freezing, put in 260Kg nitroguanidine, stir well, add 30% liquid caustic soda dropwise until the pH is 10, heat up to 45°C, and react After 2 hours, cool to room temperature, shake and filter to obtain a wet product, and dry to obtain a 243Kg finished product (more than 99% content).
Embodiment 3
[0022] Pump 150Kg ethylenediamine and 300Kg water into a 1000L reactor, add concentrated sulfuric acid dropwise to neutrality under freezing, put in 260Kg nitroguanidine, stir evenly, add 30% liquid caustic soda dropwise until the pH is 8, heat up to 80°C, and react After 10 hours, cool to room temperature, shake and filter to obtain a wet product, and dry to obtain a 230Kg finished product (more than 99% content).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com