New chiral columns with broad chiral selectivity
A chiral stationary phase and compound technology, applied in the field of chiral chemistry, can solve problems such as aggravated separation of enantiomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
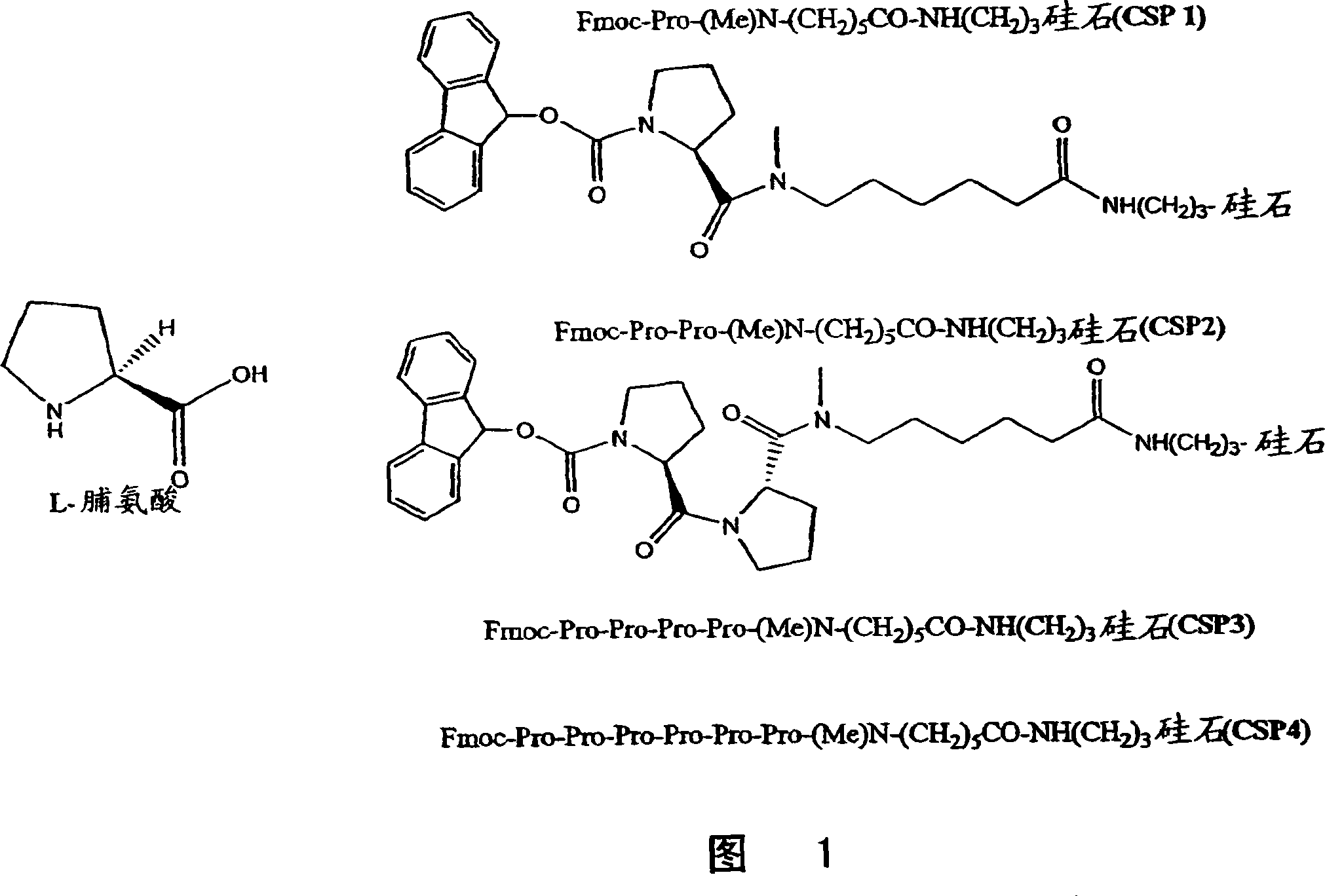
[0041] Embodiment 1: Preparation of chiral stationary phase Fmoc-Pro-(Me)Ahx-APS (CSPI)
[0042] To 0.80 g of (Me)Ahx-APS silica (surface (Me)Ahx concentration is 0.64 mmol / g) was added Fmoc-Pro-OH (3 eq., 0.52 g) in 8 mL DMF, HATU (3 eq. , 0.58 g) and DIPEA (3 equiv, 0.20 g). After stirring for 6 hours, the resulting silica was collected by filtration and washed with DMF, methanol and DCM to obtain the desired chiral stationary phase. The surface Pro concentration was determined to be 0.57 mmol / g according to the Fmoc split method. The resulting chiral stationary phase was loaded into a 50 x 4.6 mm HPLC column using the standard homogenate packing method.
Embodiment 2
[0043] Embodiment 2: the preparation of chiral stationary phase Fmoc-Pr02-(Me)Ahx-APS (CSP2)
[0044] To 0.80 g of (Me)Ahx-APS silica (surface (Me)Ahx concentration is 0.64 mmol / g) was added Fmoc-Pro-OH (3 eq., 0.52 g) in 8 mL DMF, HATU (3 eq. , 0.58 g) and DIPEA (3 equiv, 0.20 g). After stirring for 6 hours, the resulting silica was collected by filtration and washed with DMF, methanol and DCM. The surface Pro concentration was determined to be 0.55 mmol / g according to the Fmoc split method. The Fmoc protecting group was then removed by treatment with silica in DMF containing 10 mL of 20% (V / V) piperidine for 1 hour. The deprotected silica Pro-(Me)Ahx-APS was collected by filtration and washed with DMF, methanol and DCM. Another module, Fmoc-Pro-OH, was then incorporated onto the resulting silica after the same sequence of reactions to obtain the desired chiral selector on silica. The surface Fmoc concentration was determined to be 0.52 mmol / g according to the Fmoc splitt...
Embodiment 3
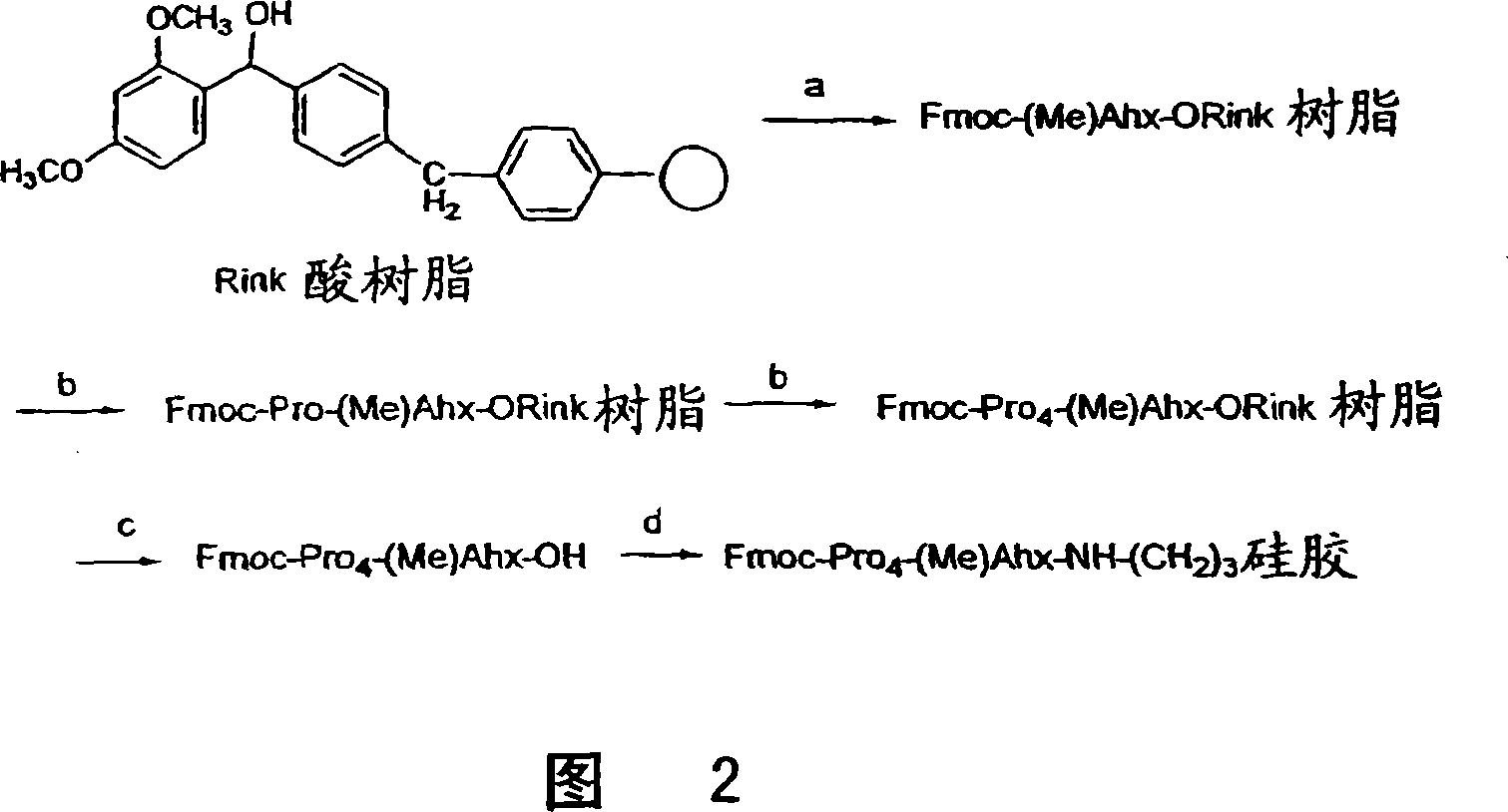
[0045] Example 3: Preparation of chiral stationary phase Fmoc-Pro4-(Me)Ahx-APS(CSP3)
[0046] To Rink acidic resin (100-200 mesh, 3.0 g, 0.43 mmol / g) pre-swelled with DCM (20 mL, 30 min) was added Fmoc-(Me )A mixture of Ahx-OH (1.42g, 3.87mmol), DMAP (0.16g, 1.29mmol), NMM (0.39g, 3.87mmol), and DIC (0.49g, 3.87mmol). After stirring for 6 hours, the resin was collected by filtration and washed with DMF, DCM and methanol (20 mL x 3). The Fmoc protecting group was removed by treatment with 20 mL of 20% (V / V) piperidine in DMF for 30 minutes. The deprotected (Me)Ahx-O-Rink resin was collected and washed with DMF, DCM and methanol (20 mL x 3).
[0047] To (Me)Ahx-O-Rink resin was added Fmoc-Pro-OH (1.31 g, 3.87 mmol), HATU (1.47 g, 3.87 mmol), and DIPEA (0.50 g, 3.87 mmol) in 20 mL of anhydrous DMF mixture. After stirring for 3 hours, the resin was filtered and washed with DMF, DCM and methanol (20 mL x 3).
[0048] Then the Fmoc group was removed, and the second, third and f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com