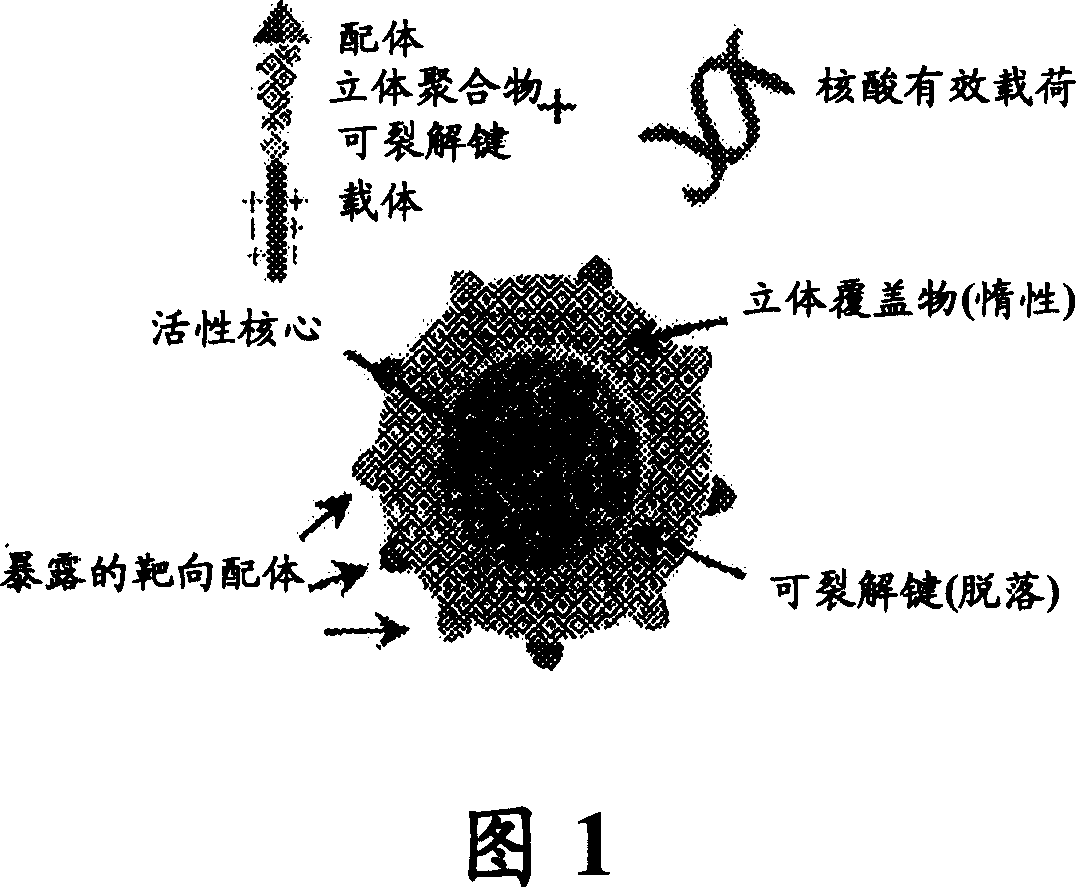
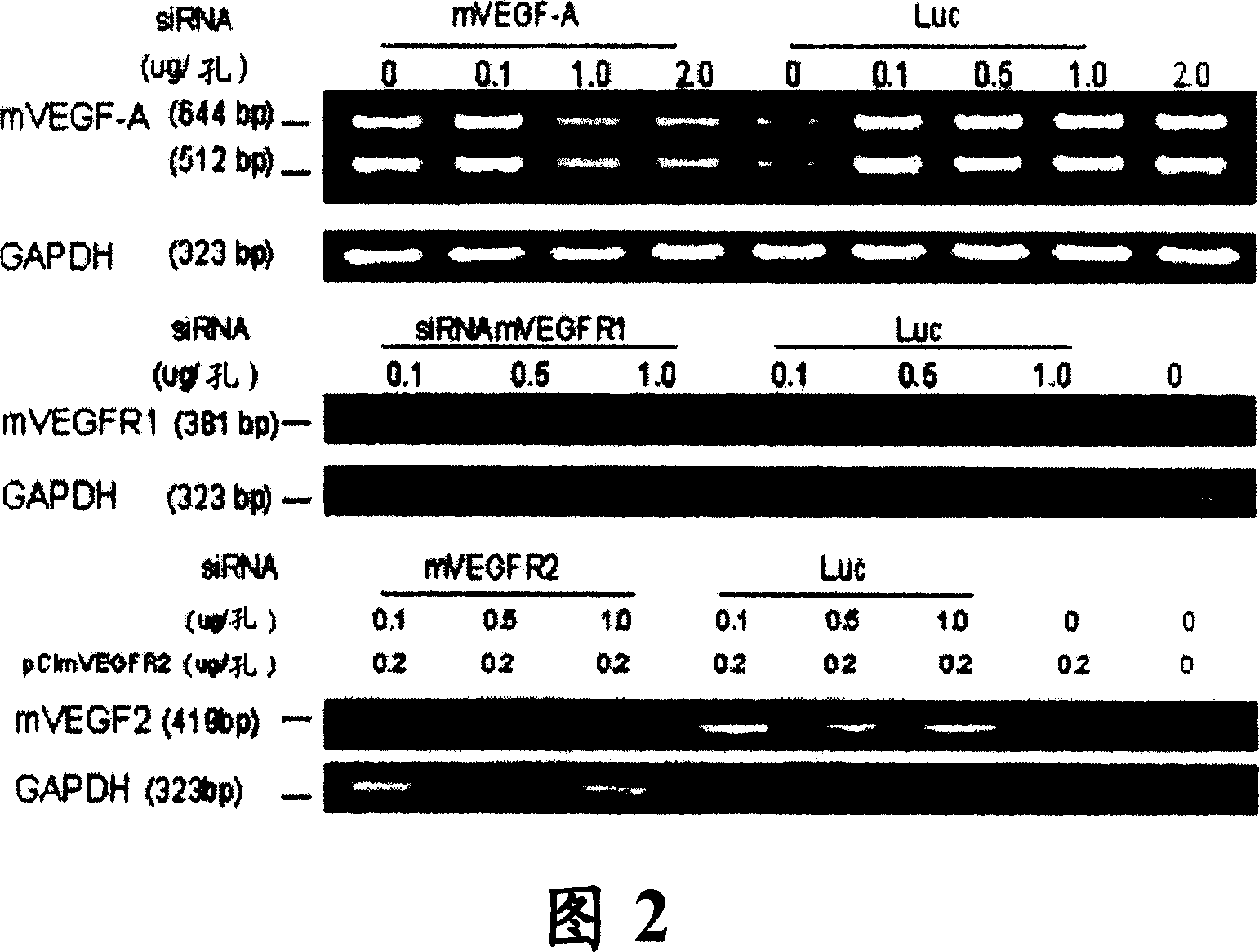
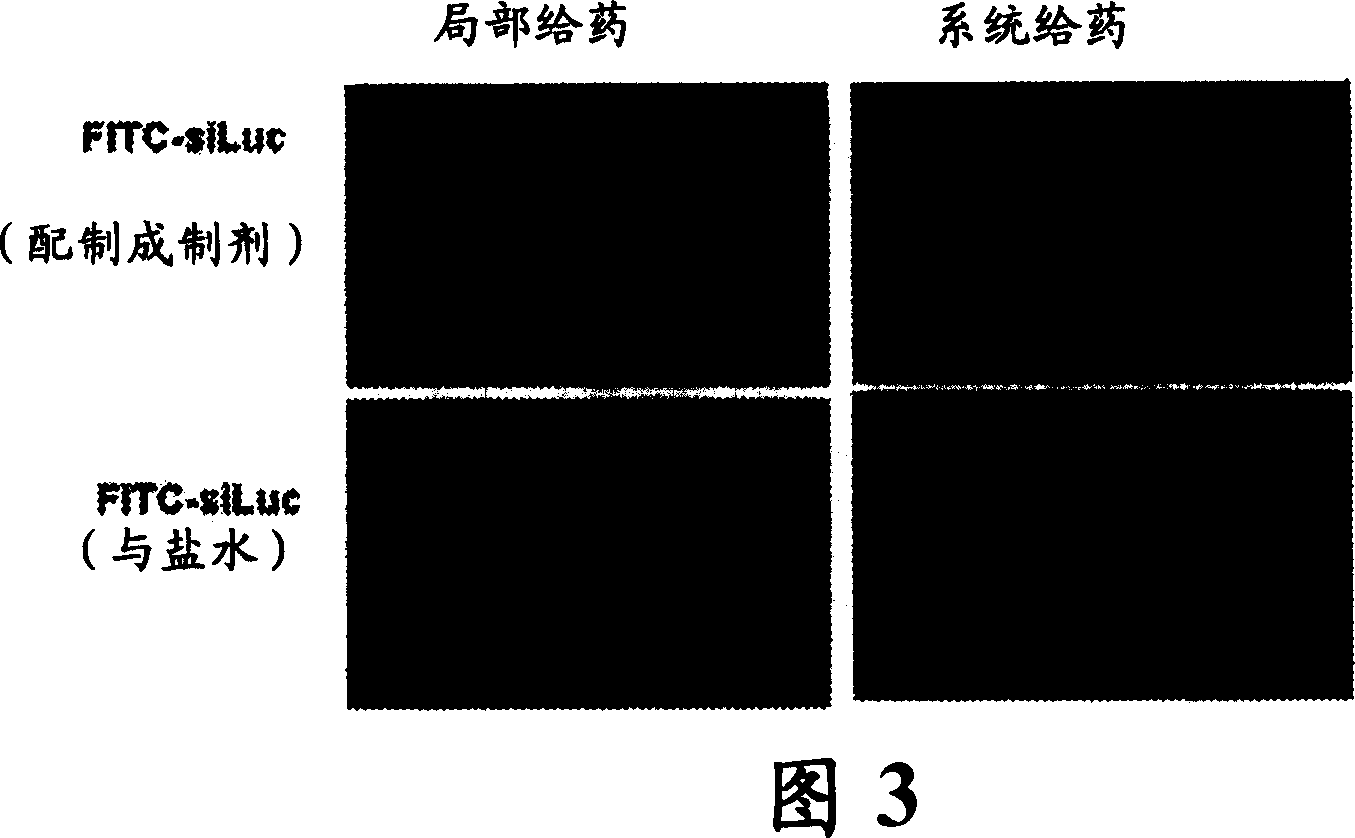
RNAi therapeutics for treatment of eye neovascularization diseases
A new blood vessel, eye disease technology, applied in DNA/RNA fragments, drug combinations, sugar derivatives, etc., can solve problems such as failure to work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1. Topical and Systemic Administration of VEGF Pathway Inhibitors to Treat Anterior Eye NV Disease Mouse SK Model, Virus and Tissue Culture
[0083] An ocular stromal keratitis (SK) BALB / c mouse model has been reported in which corneal NVs were implanted into the stroma by a micropouch approach with CpG DNA oligonucleotides (CpG ODN, containing NV-inducible motif equivalents of the HSV DNA genome) Induction, or HSV-1 virus infection by corneal scratch method to induce. The sequences of the stimulating ODN used in this study are: 1466, TCAACGTTGA and 1555, GCTAGA CGTTAGCGT (provided by Dr. Dennis M. Klinman, Center for Biologics Evaluation and Research, US FDA). Microspheres for implantation into corneal micropockets contained an equimolar mixture of ODN 1466 and 1555 and a previously reported hydron polymer. Use HSV-1 RE strain (provided by Dr. Robert Lausch of Uni.Alabama, Mobile) with 2-μl 1×10 per eye 5 Doses of plaque-forming units induce HSK. To test the...
Embodiment 2
[0140] Example 2. Topical Administration of VEGF Pathway Inhibitors for Posterior Ocular NV Disease
[0141] Materials and methods
[0142]Pups of the same surrogate mother were treated with hypoxia (75%) from P7 to P12 and switched to normal air (normoxia) from P12 to P16. (P number refers to the number of days). Subconjunctival administration of siRNA complexed with the cationic polymer reagent PolyTran PT73 was employed. The ratio of siRNA to PT73 was 1:8 by weight. The mixture was diluted to the required volume with 5 mM HEPES solution. The siRNA dose was 4 μg siRNA (in PT73 complex) per eye dispersed in a volume of 5 μl. One injection each at P12 and P13. The left eye of each mouse was treated with negative control siRNA, siLuc, and the right eye was treated with active siRNA, siMix. Negative control siLuc was an equal mixture of two oligonucleotides (siLuc-a and b). siMix is an equal mixture of simVEGFA, simVEGFR1 and simVEGFR2, each of which is a mixture of two...
Embodiment 3
[0145] Example 3. Distal Systemic Administration of VEGF Pathway Inhibitors to Treat Novel Tumor Model Systems
[0146] Angiogenesis
[0147] Materials and methods
[0148] nucleic acid
[0149] Based on the study by Elbashir et al. (2), short double-stranded RNA oligonucleotides for siRNA-tagged siLuc, siLacZ, siGFP, and siVEGFR2 were designed, lacking significant interfering homology as confirmed by BLAST analysis, and synthesized by Dharmacon (Lafayette, CO) and purification. Two sequences were synthesized per target, combined in a 1:1 molar ratio. 所用的靶序列为,siLuc:aaccgctggagagcaactgca和aagctatgaaacgatatgggc,siLacZ:aacagttgcgcagcctgaatg和aacttaatcgccttgcagcac,siGFP:aagctgaccctgaagttcatc和aagcagcacgacttcttcaag,siVEGFR2:aatgcggcggtggtgacagta和aagctcagcacacagaaagac(已描述了此siRNA对VEGF R2的抑制作用(28))。 siRNA targeting luciferase was labeled with luciferin at the 3' position of the sense strand by standard bond chemical conjugation (FITC-siRNA) for FACS analysis and tissue distribution ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com