Application of specific identification tau antibody, and reagent kit containing thereof
A technology for specific identification and diagnostic kits, which can be used in testing pharmaceutical preparations, biological tests, anti-animal/human immunoglobulins, etc., and can solve problems such as omissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1: Elevated tau levels in children with leukemia
[0096] To evaluate the effect of chemotherapy on neuronal damage, an in-depth study was performed that included 65 leukemic children (2 to 16 years) treated according to standard procedures, without measurable central nervous system lesions. A total of 377 CSF samples were analyzed. Before each injection, a small amount of fluid was collected for routine laboratory analysis and the remainder was used in our study. The children were diagnosed and treated for leukemia at the Leuven University Hospital in Belgium. Tau protein in cerebrospinal fluid was assessed using INNOTEST hTAU antigen (Gent Innogenetics, Belgium).
[0097] Before starting treatment, perform a lumbar puncture in all children suspected of having leukemia to detect the possible presence of leukemic cells, an indication of central nervous system infiltration. The tau levels measured at this time, ie, before treatment, serve as control levels to ...
Embodiment 2
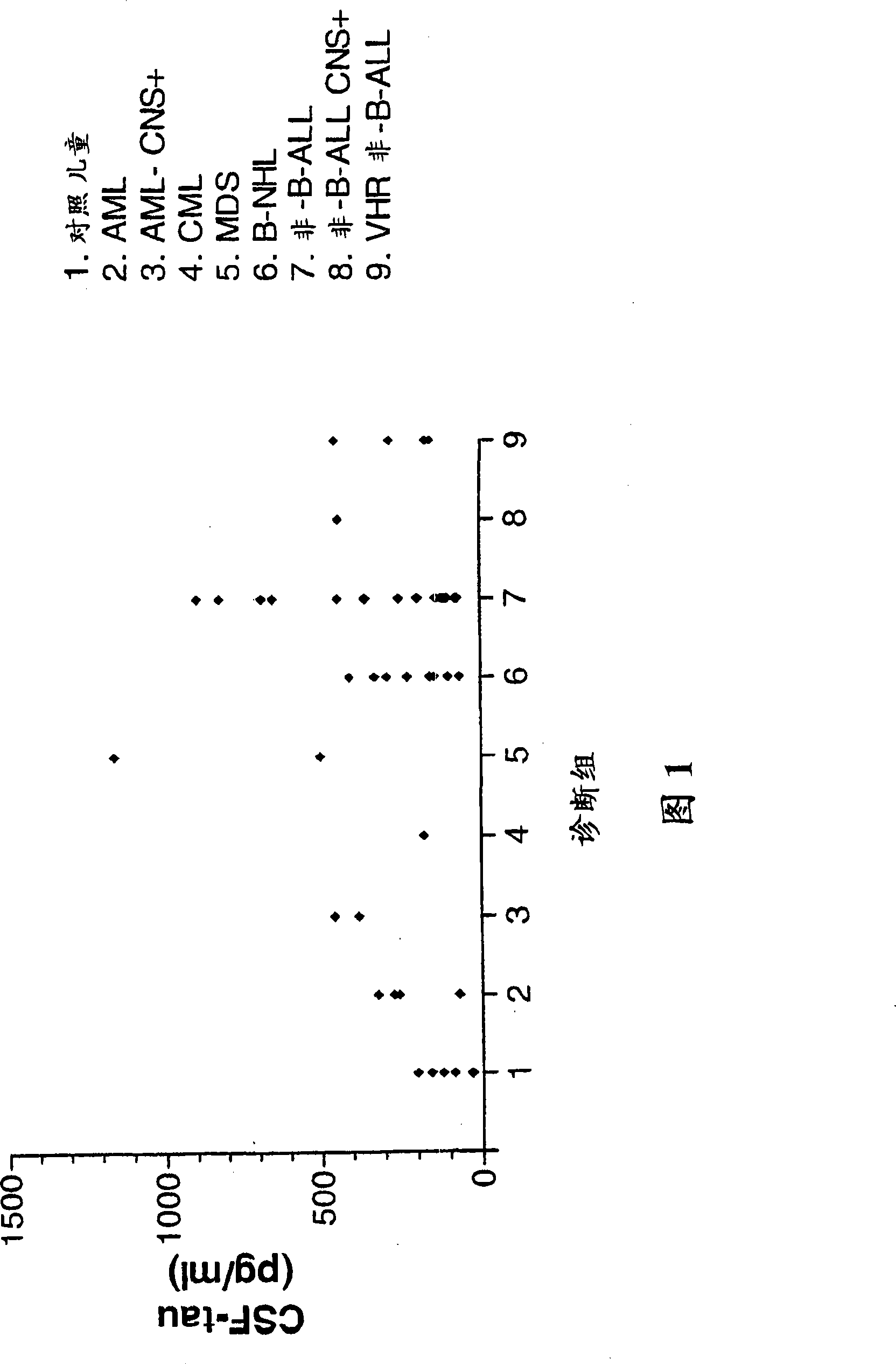
[0099] Example 2: Elevated levels of tau in leukemia patients before treatment
[0100] 1. Research object
[0101] Between August 1996 and June 1999, 510 CSF samples were collected from 82 children receiving cancer treatment at the Children's Hematology-Oncology Unit of the Catholic University in Leuven, Belgium. CSF samples were only taken during lumbar puncture (LP) scheduled for malignancy staging or treatment.
[0102] Three groups of patients with hematologic malignancies were studied. The largest cohort consisted of 48 non-B-ALL patients treated according to EORTC protocol 58881 (Table 1). Of these children, 20 had CD10(+) blasts (or common ALL), 2 of whom also had Down syndrome (DS), 1 had Brachmann-de Lange syndrome; 6 had common 2 patients had normal T-blasts, 2 patients had pro-B-blasts, 9 patients had pre-B-blasts, and 9 patients had T-blasts. Forty-two children had leukemia, and six patients had stage II (one), III (four), or IV (one) non-Hodgkin's lymphoma. ...
Embodiment 3
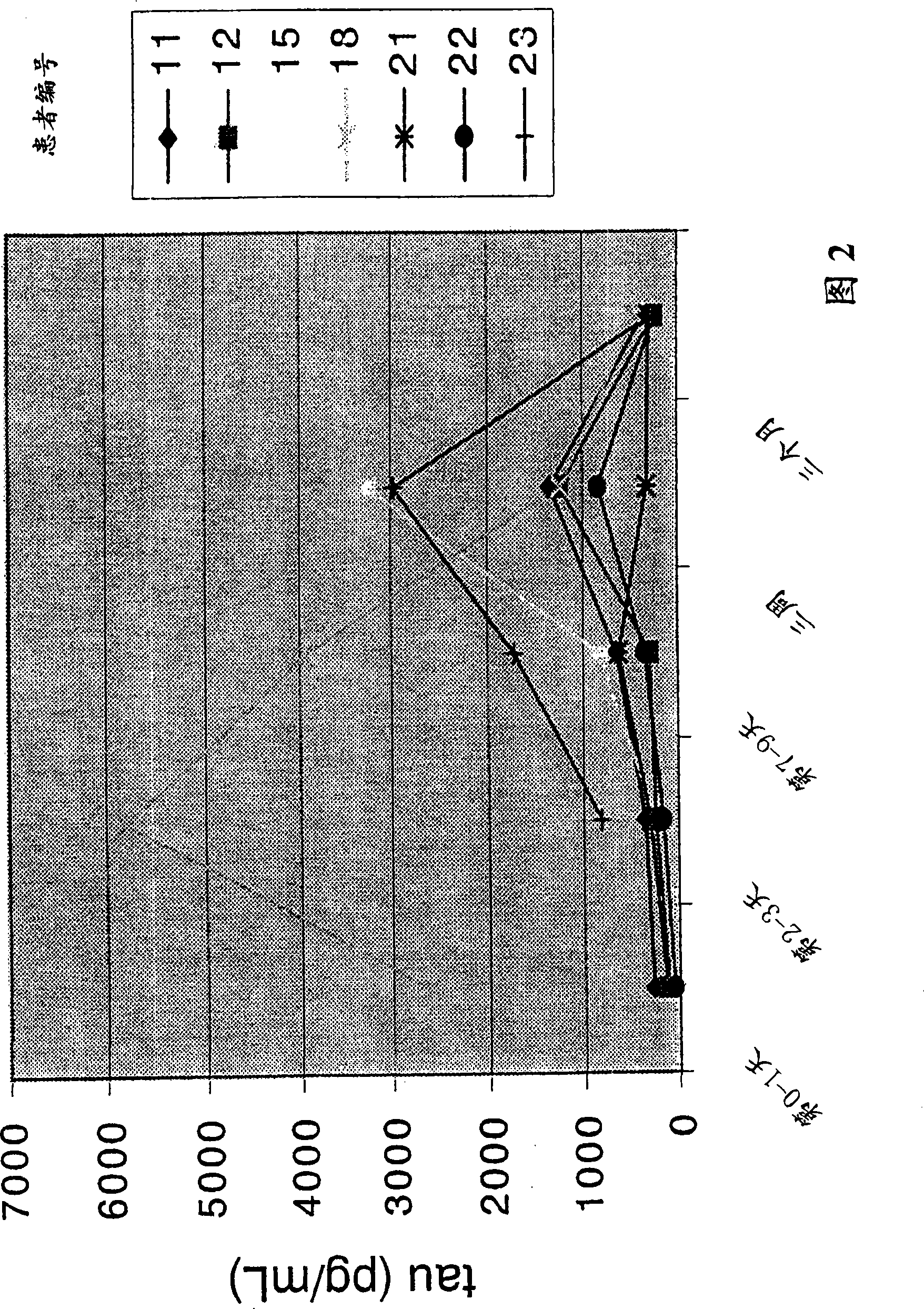
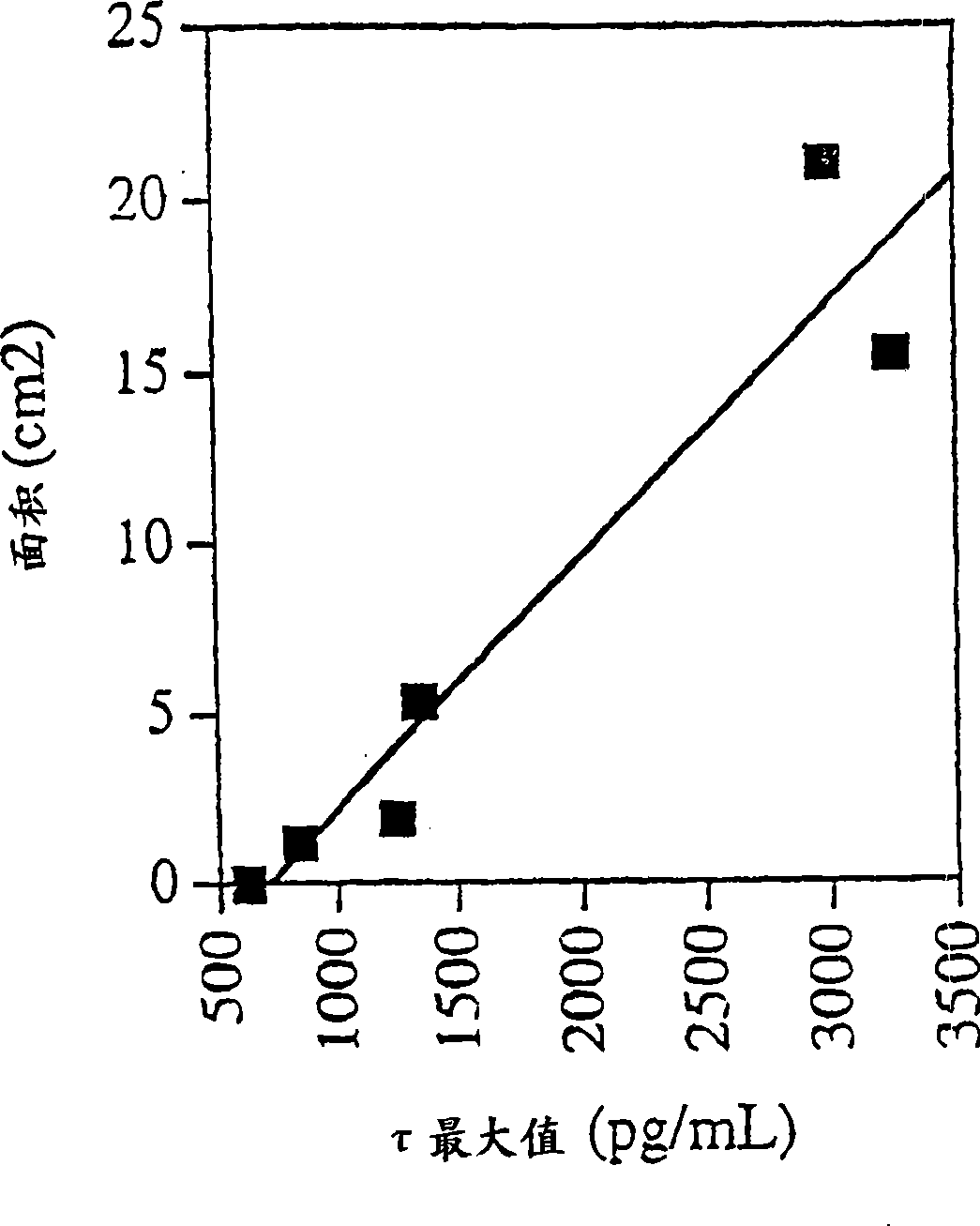
[0113] Example 3: Use of CSF-τ as a marker for early detection of possible CNS damage from stroke
[0114] 1. Research object
[0115] Seven patients participated in the study, 3 males and 4 females, aged 63-81 (mean SD, 70.7±7.2 years old), suffering from cerebral infarction, admitted to Sweden Neurology ward at Sahlgren University Hospital. All patients were enrolled in the study within 72 hours of stroke onset.
[0116] 2. Method
[0117] CSF samples were collected by lumbar puncture. Collect 12 ml and freeze each 0.5 ml at -80°C until analysis. CSF samples were collected on admission (day 0-1), day 2-3, day 7-8, day 21-22 (3 weeks), and day 90-110 (3 months). CSF-τ levels were determined by sandwich ELISA (INNOTEST ThTAU antigen, Gent Innogenetics Ltd., Belgium), measuring total tau (normal and hyperphosphorylated tau).
[0118] Brain computed tomography (CT) was used to check the extent of brain damage on the first day of admission. The modified Scandinavian Stro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com