Quassia wood total alkaloid and the medical function of the monocase alkaloid
A technology of application and compound of general formula, applied in the field of medical use of total alkaloids and monomer alkaloids of Momordica fragrans, and can solve problems such as no report on inhibition effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0037] Preparation Example 1: Preparation of Total Alkaloids
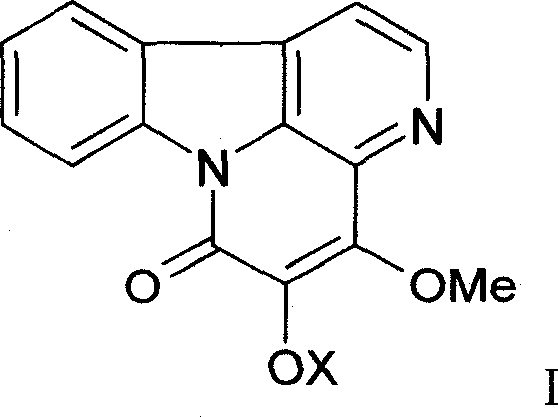
[0038] After crushing 1 kg of bitter wood medicinal material, heat and extract with 10 liters of 75% ethanol twice, each time for 2 hours, combine the extracts, recover the ethanol under vacuum at 60°C, adjust the pH value to 11 with concentrated ammonia water, and extract with an equal volume of ethyl acetate 2 times, the extracts were combined, ethyl acetate was recovered, the residue was dissolved with 0.5% hydrochloric acid, and the above step of adjusting the pH with concentrated ammonia water—ethyl acetate extraction was repeated to finally obtain 17.1 g of total alkaloids. High performance liquid phase (HPLC) method is measured, and 4,5-dimethoxy ferric ketone, 4-methoxyl-5-hydroxyl ferric ketone both total content is 52% by weight / weight (w / w).
preparation example 2
[0039] Preparation example 2: the preparation of the total alkaloids of bitter wood
[0040]After crushing 1 kg of bitter wood medicinal material, heat and extract with 10 liters of 75% ethanol twice, each time for 2 hours, combine the extracts, recover the ethanol under vacuum at 60°C, adjust the pH value to 11 with concentrated ammonia water, and extract with an equal volume of ethyl acetate 2 times, the extracts were combined, ethyl acetate was recovered, the residue was dissolved with 0.5% hydrochloric acid, and the above step of adjusting the pH with concentrated ammonia water—ethyl acetate extraction was repeated to finally obtain 17.1 g of total alkaloids. 600g of column chromatography silica gel (200-300 mesh) is wet-packed with chloroform, 10g of total alkaloids are dissolved in absolute ethanol, mixed with 15g of column chromatography silica gel (100-120 mesh), and then mixed with chloroform after loading Gradient elution with methanol (the initial solvent is chlorof...
preparation example 3
[0041] Preparation Example 3: Preparation of 4,5-dimethoxyfermidone and 4-methoxy-5-hydroxyferricone
[0042] 600g of column chromatography silica gel (200-300 mesh) is wet-packed with chloroform, 10g of total alkaloids are dissolved in absolute ethanol, mixed with 15g of column chromatography silica gel (100-120 mesh), and then mixed with chloroform after loading Gradient elution with methanol (the initial solvent is chloroform), 250ml was collected once, checked by TLC, the same items were combined, and silica gel column chromatography was repeated to obtain 2.1g of 4,5-dimethoxyferricone, which was normalized by HPLC Check the purity of 98.7%; 1.7 g of 4-methoxy-5-hydroxyferricone can be obtained, and the purity of HPLC normalized check is 98.2%. , the physical and chemical properties are as follows: yellow needle crystal (acetone), mp 145 ~ 146 ° C, bismuth potassium iodide reaction was positive. IR v max KBr cm -1 : 1670, 1635, 1270, 1110, 1090. 1 H-NMR (400MHz, DMSO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com