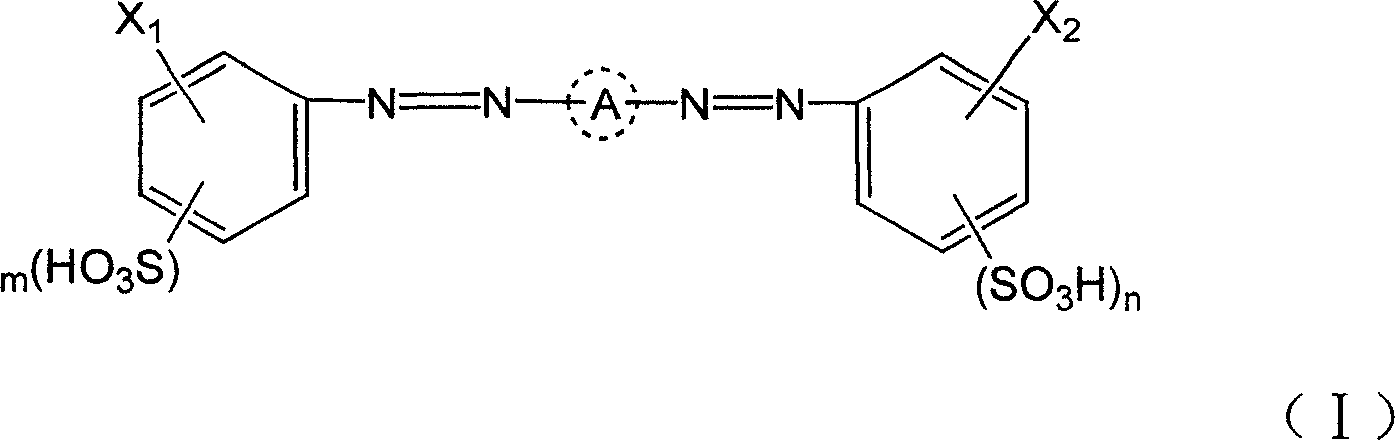
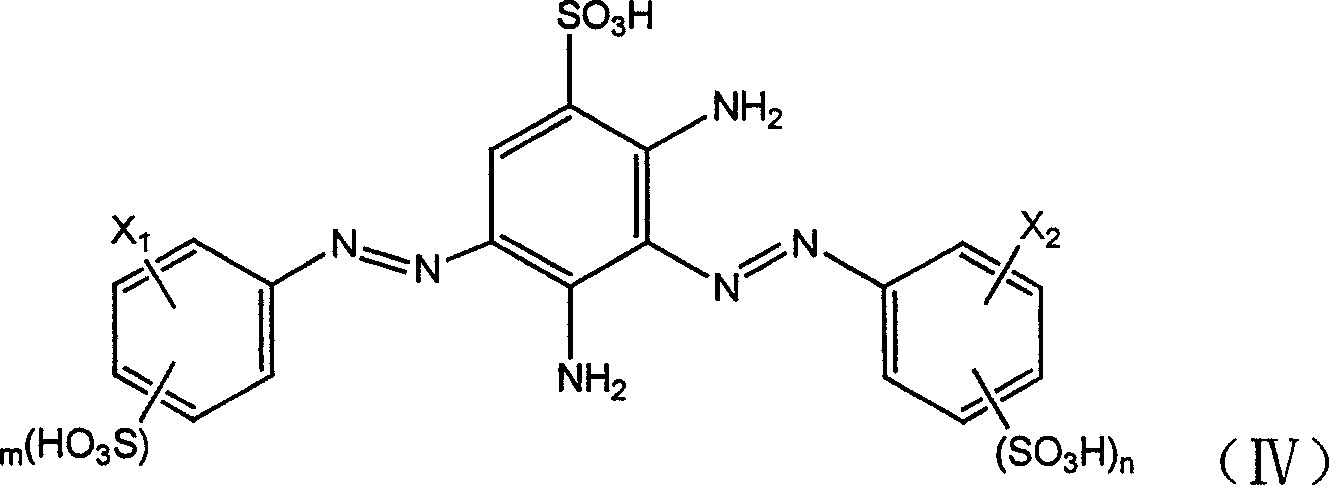
Bi-azo active dye, its production and composition
A technology of reactive dyes and disazo, applied in azo dyes, reactive dyes, dyeing methods, etc., can solve problems affecting economy and environmental protection, unusable hydrolyzed dyes, poor compatibility, etc., and achieve bright color and light , Excellent application effect, high blackness effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 24.3 parts of substituted aniline of formula (VI-a), add 100 parts of water and stir evenly, cool to 0-5°C, add 18 parts of concentrated hydrochloric acid, and add 6.9 parts of sodium nitrite with a content of 30% dropwise at 0-5°C Add the aqueous solution within 10 minutes, continue to stir for 20 minutes, add sulfamic acid to eliminate excess nitrous acid, and obtain diazonium salt.
[0053] Add 18.8 parts of m-phenylenediamine o-sulfonic acid into 100ml of water and stir, adjust the pH to 7, after dissolving, add dropwise to the diazonium salt of formula (VI-a), the coupling temperature is 10°C, and adjust the pH of the solution to 2.5- 3. The dropwise addition is completed within 30 minutes. After the dropwise addition, adjust the pH of the solution to 2.5-3 with 10% sodium carbonate solution and react for 3 hours to obtain the monoazo dye of formula (VII-a).
[0054] Add 38.3 parts of the sulfonated para-ester of formula (VIII-a) to 100ml of water and stir, add 18m...
Embodiment 2~8
[0058] According to the preparation method of Example 1, the difference is that the compounds listed in Table 1 are used to replace formula (VI-a) and formula (VIII-a) in Example 1 respectively, and a series of orange dyes as shown in the following table can be obtained.
[0059] Table 1
[0060]
[0061]
[0062]
Embodiment 9
[0064] 24.3 parts of substituted aniline of formula (VI-a), add 100 parts of water and stir evenly, cool to 0-5°C, add 18 parts of concentrated hydrochloric acid, add 6.9 parts of 30% aqueous solution of sodium nitrite dropwise at 0-5°C, The addition was completed within 10 minutes, and after continuing to stir for 20 minutes, sulfamic acid was added to eliminate excess nitrous acid to obtain diazonium salt.
[0065] Add 21.58 parts of 2-amino-5-naphthol-7-sulfonic acid into 100ml of water and stir, adjust the pH to 7 and dissolve, then add dropwise to the diazonium salt solution of formula (VI-a) obtained above, the coupling temperature At 10°C, adjust the pH of the solution to 2-2.5 at the same time, and complete the dropwise addition within 30 minutes. After the dropwise addition, adjust the pH of the solution at 2-2.5 with 10% sodium carbonate solution and react for 3 hours to obtain the monoazo dye of formula (VII-b) .
[0066] Add 38.3 parts of the sulfonated para-ester...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com