Novel use of chiral (R/S)-a-phenethylamine(+/-)-tartrate
A tartrate, a new-purpose technology, applied in the field of known compounds, can solve the problems of complicated operation and low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] R / S-a-phenylethylamine: produced by Changzhou Kerunda Technology Co., Ltd.
[0024] (+ / -)-tartaric acid: produced by Hangzhou Linan Jinlong Chemical Co., Ltd.
[0025] (1) Preparation of chiral catalyst
[0026] 1a: Preparation of R-a-phenethylamine-(-)tartrate
[0027] Preparation of R-a-phenylethylamine-(-)tartaric acid salt
[0028] In a 250ml Erlenmeyer flask, add 6.3g (-)-tartaric acid and 90ml methanol, heat on a water bath to near boiling (about 60°C), and stir to dissolve the tartaric acid. Then, 5 g of R-α-phenylethylamine was slowly added under stirring. Placed for more than 24h, white prismatic crystals. Suction filter, wash with a small amount of cold methanol, and dry to obtain R-amine·(-)-tartrate.
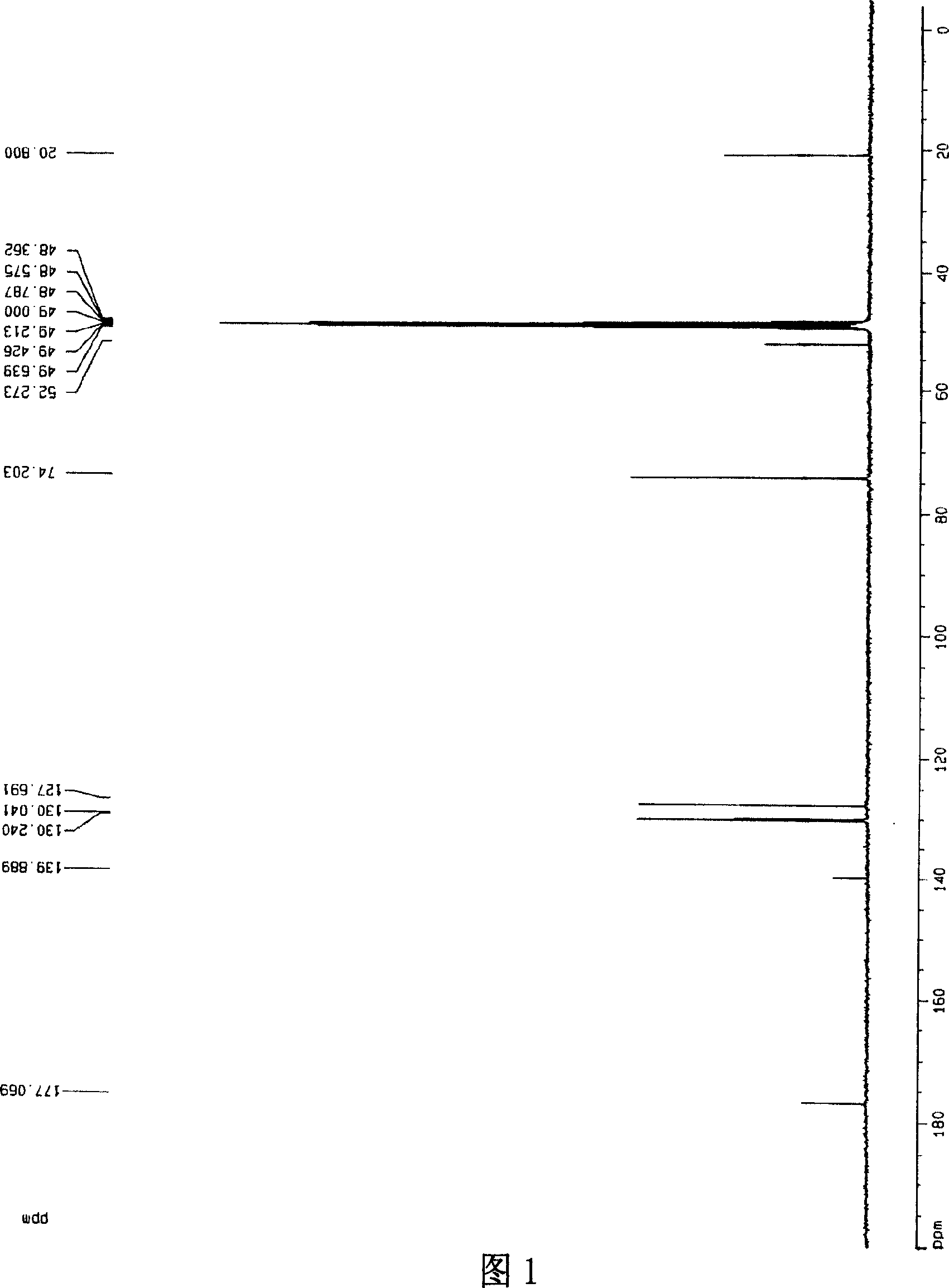
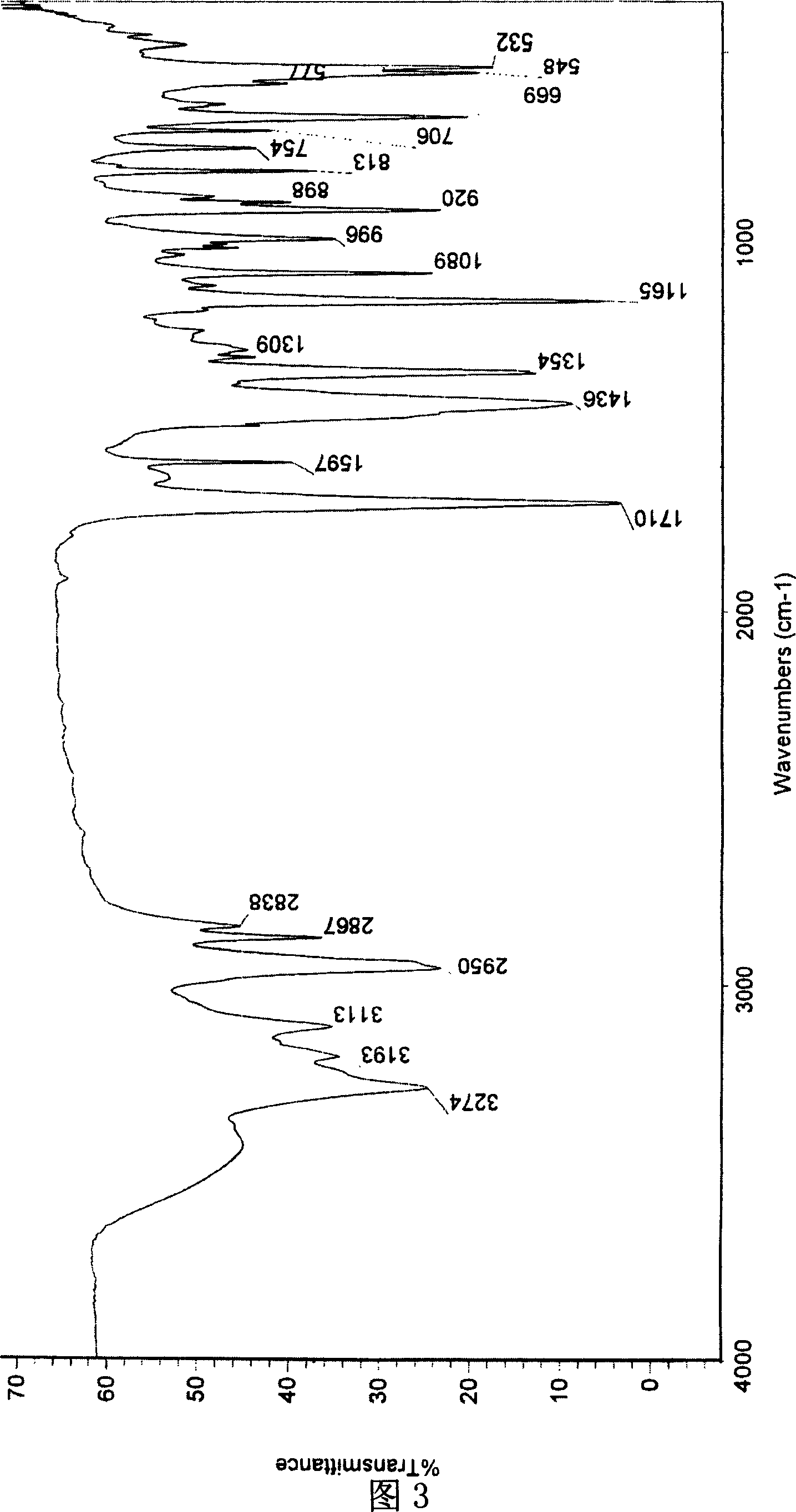
[0029] [a] 5 D =-10.1° (c=1.30, CH 3 OH); 1 HNMR (300MHz, CD 3 OD, 27℃), δ(ppm)=7.38~7.48(m, 5H), 4.89(s, 5H), 4.43~4.48(m, 1H), 4.40(s, 2H), 1.62~1.64(d, J =5.16,3H), 13 CNMR, 20.80(x2), 52.27, 74.20, 127.69, 130.04, 130.24, 139.89, 177.07, .IR: 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com