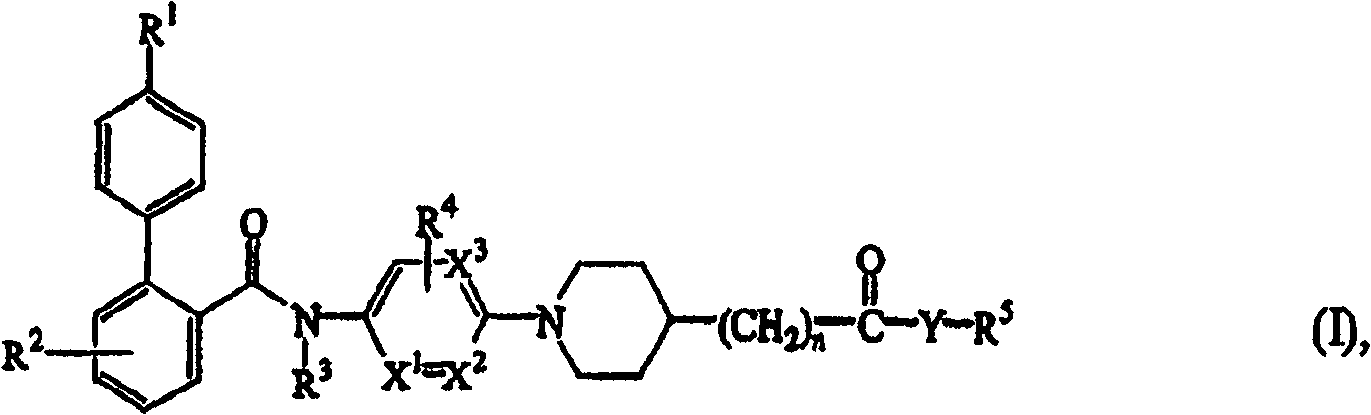
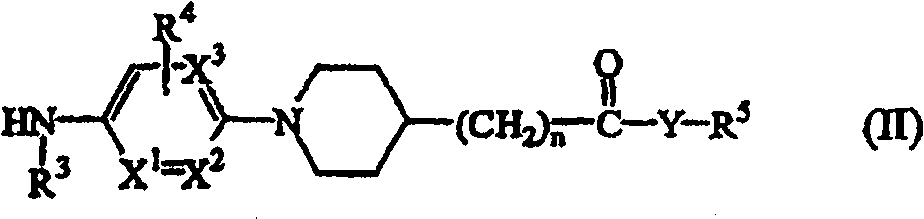
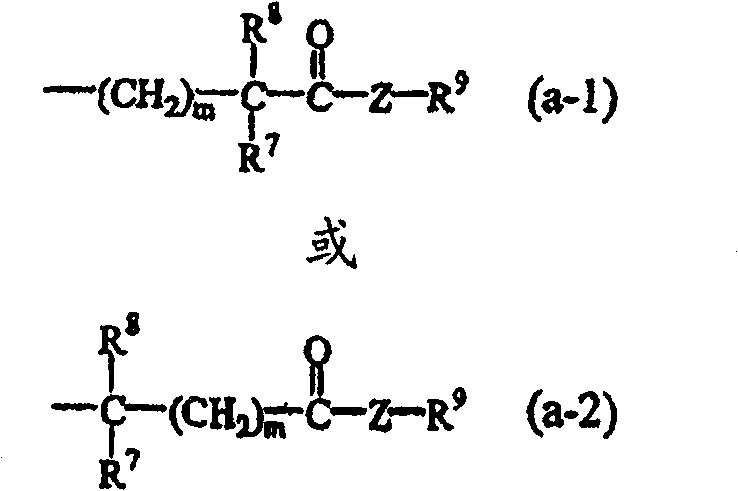N-aryl piperidine substituted biphenylcarboxamides as inhibitors of apolipoprotein B
An aryl and alkoxycarbonyl technology, applied in the field of new N-arylpiperidine substituted biphenyl carboxamides, can solve the problem of limited drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
[0165] Preparation of intermediate (1)
[0166] At 0°C, 4'-(trifluoromethyl)-[1,1'-biphenyl]-2-carboxylic acid (0.0069mol) was dissolved in dichloromethane (400ml) and oxalyl chloride (0.069mol) and di methylformamide (1 drop). Under nitrogen flow, 4'-(trifluoromethyl)-[1,1'-biphenyl]-2-carboxylic acid (0.0621 mol) was added in portions again. Oxalyl chloride (0.069 mol) and dimethylformamide (1 drop) were added and the reaction mixture was stirred at 0°C for 2 hours. The reaction mixture was filtered, the residue was dissolved in dichloromethane (100ml), and the resulting mixture was added dropwise to 1-(4-aminophenyl)-4-piperidine ethyl acetate (0.069mol), tris A mixture of ethylamine (17.5ml) and dichloromethane (300ml). The reaction mixture was allowed to reach room temperature over 90 minutes. The resulting reaction mixture was washed with water, dried and the solvent was evaporated. The residue was stirred in a hot mixture of hexane and ethyl acetate and the resul...
Embodiment A2
[0170] Preparation of intermediate (3)
[0171] At 0°C, 6-methyl-4'-(trifluoromethyl)-[1,1'-biphenyl]-2-carboxylic acid (0.0025mol) was dissolved in anhydrous dichloromethane (140ml) and ethyl alcohol diacid dichloride (2.4ml) and a few drops of dimethylformamide. The remainder of 6-methyl-4'-(trifluoromethyl)-[1,1'-biphenyl]-2-carboxylic acid (0.0225 mol) was then added in portions under nitrogen flow. The reaction mixture was heated gently to 40 °C until a homogeneous solution was obtained and gas evolution ceased. The mixture was allowed to cool to room temperature, then filtered through a Buchner filter. The filter residue was dissolved in dichloromethane, and then added dropwise to an anhydrous solution of ethyl 1-(4-aminophenyl)-4-piperidine acetate (1 equivalent, 0.025 mol) and triethylamine (3 g) at 0 ° C. Dichloromethane (140ml) solution. The reaction mixture was allowed to warm to room temperature over 90 minutes. The precipitate was filtered off and dried to ...
Embodiment A3
[0175] Preparation of intermediate (5)
[0176] A mixture of ethyl 4-piperidinecarboxylate (0.03mol), 1-fluoro-4-nitro-benzene (0.03mol), potassium carbonate (4.5g) and dimethylformamide (50ml) was reacted at 60°C for 4 hours , then the solvent was evaporated under reduced pressure, and the resulting residue was stirred in water. The resulting yellow solid was filtered off and dissolved in dichloromethane (100ml). The organic layer was dried and the solvent was evaporated. The resulting oil was crystallized from hexane, and the desired product was collected to give 7 g of intermediate (5) (m.p. 70-71°C).
[0177] Preparation of intermediate (6)
[0178] At 25 °C, a mixture of intermediate (5) (0.025 mol) and ethanol (150 ml) was heated under pressure (30 bar = 3.10 6 Hydrogenation overnight at Pa) over catalyst Pd / C 10% (0.5 g). After uptake of hydrogen (3 equivalents), the reaction mixture was filtered and the solvent was evaporated under reduced pressure to yield 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com