Amphioxus galactose lectin AmphiGAL13-gene and its use
A technology of gene and gene coding, which is applied in the fields of application, genetic engineering, plant genetic improvement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Extraction of total RNA from the digestive tract of amphioxus and synthesis of cDNA
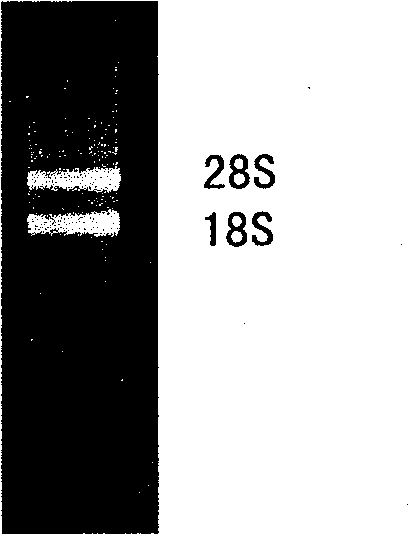
[0029]Extraction of total RNA and synthesis of cDNA; the digestive tract of amphioxus was collected, the total RNA of the digestive tract was extracted by Trizol reagent, and the protein was extracted by phenol / chloroform extraction, and the total RNA of the digestive tract of amphioxus was obtained. 260 / A 280 = 1.828, two clear bands of 18s and 28s can be seen through 1% formaldehyde denaturing gel electrophoresis detection, the ratio> 1 (see figure 2 ), indicating that the total RNA has better integrity and higher purity. Take 1ug of total RNA, with SMART III olignuclotide (5'-AAGCAGTGGTATCAACGCAGAGTGGCCATTATGGCCGGG-3') and CDSIII / 3'PCR primer (5'-ATTCTAGAGGCCGAGGCGGCCGACATG-d(T) 30 N -1 N-3') was reverse-transcribed to synthesize the first strand, and 10 μl of the first-strand cDNA product was obtained.
[0030] The 2ulcDNA one-strand product was taken, and the seco...
Embodiment 2
[0031] Example 2: Determination and Analysis of AmphiGAL13 Gene Sequence in Amphioxus Gastrointestinal Tract
[0032] The second-strand cDNA was connected to the pcDNA3.0 vector (purchased from Invitrogen), transformed into DH5α Escherichia coli, and the recombinant clones were selected and sequenced. Blast homology analysis showed that the EST sequence encoding the full-length AmphiGAL13 gene was obtained. The length of the gene was 465bp, encoding a Galectin protein with 154 amino acids, which was a new Galectin protein.
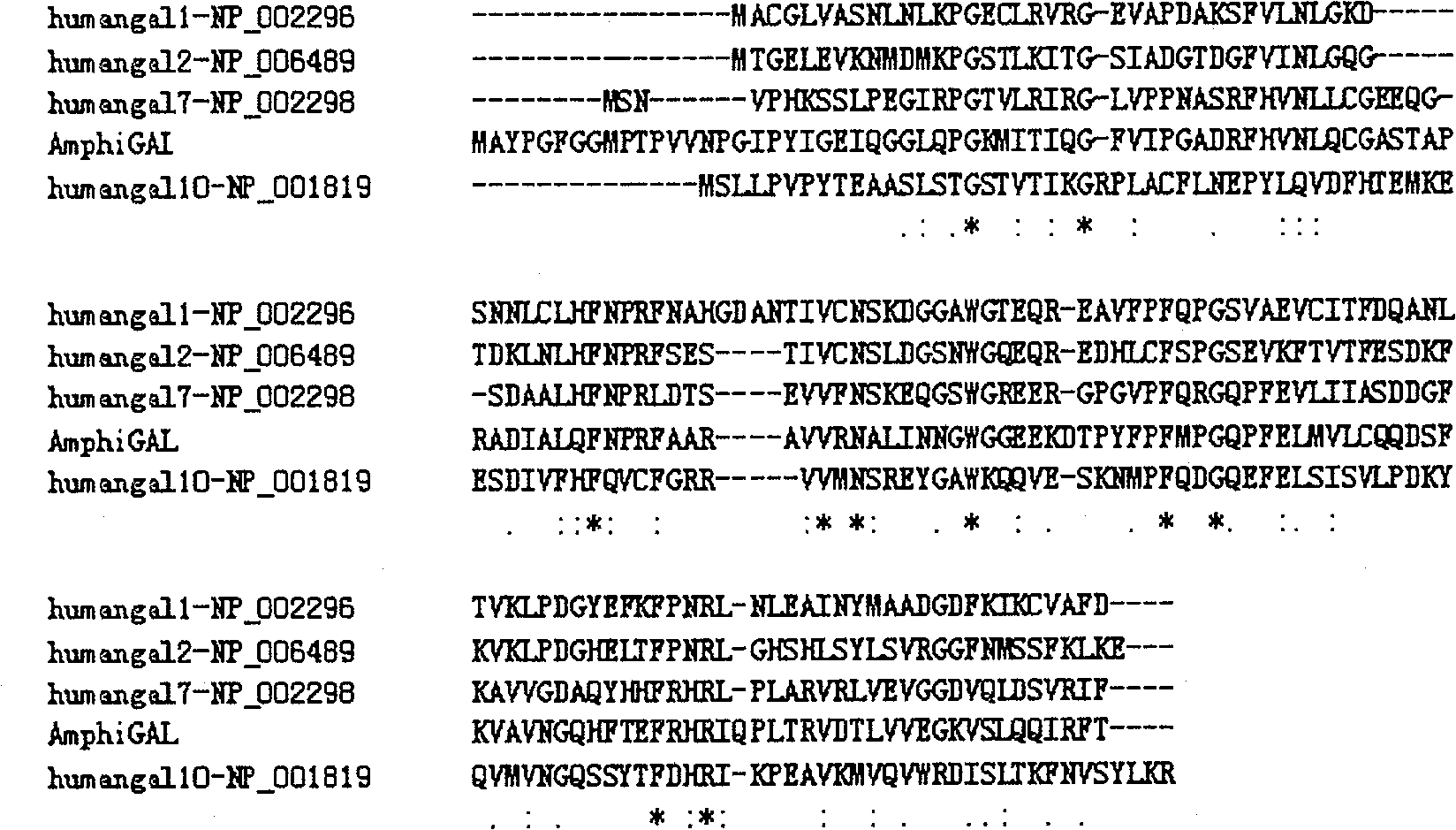
[0033] Using the ClustalW analysis software to compare the reported human prototype Galectin protein sequences, the results are as shown. From figure 1 It can be seen that the sugar recognition domain of Galectin is relatively conserved among all Galectins.
Embodiment 3
[0034] Embodiment 3: Construction of recombinant PGAL13 expression plasmid
[0035] A pair of primers were synthesized based on the sequences at both ends of the AmphiGAL3 gene, the upstream primers contained BamH I and Eenterkinase cleavage sites, and the downstream primers contained Not I cleavage sites.
[0036] Upstream primer (P1):
[0037] 5′-CG ACGACGACGACAAA ATGG CGT ACC CAG GAT T-3′;
[0038] BamH I Eenterkinase
[0039] Downstream primer (P2):
[0040] 5′-ATA AGA AT TT ATG TGA AGC GGA TCT GCT-3';
[0041] Not I
[0042] Using the pcDNA3.0 plasmid (purchased from Invitrogen) containing the AmphiGAL 13 gene as a template and P1 and P2 as primers for PCR amplification, a specifically amplified single band was obtained with a product size of about 460 bp. The PCR amplified product was cloned into the prokaryotic fusion expression vector pGEX-4T (purchased from Amersham Bioscience Company) to obtain the recombinant expression vector pGEX-AmphiGAL13 (its constru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com