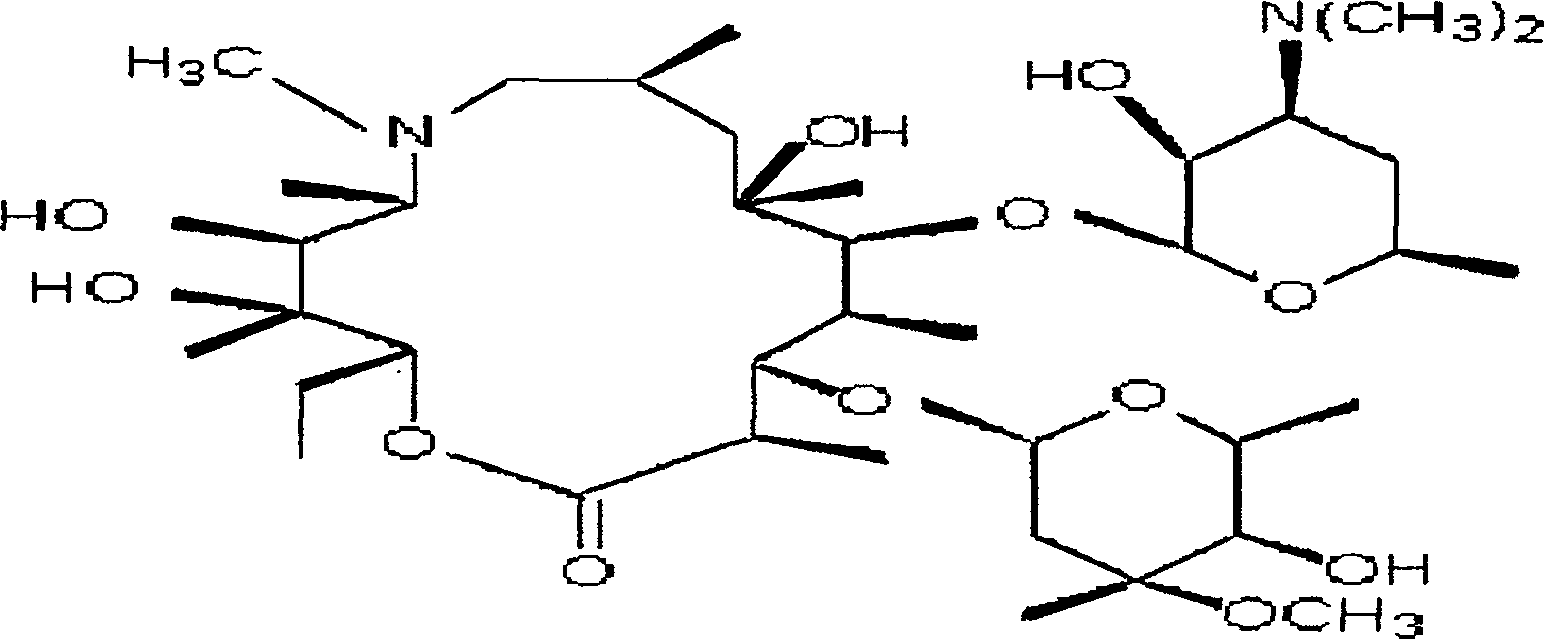
Azithromycin oral disintegration tablet and its preparing method
A technology of oral disintegrating tablets and azithromycin, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and pill delivery, etc., can solve the problem that the medication needs of all patients cannot be fully met, that it is inconvenient for patients to swallow and take water, and it is difficult for patients to take medicines, and children cannot take medicines by themselves. and other problems to achieve the effect of improving bioavailability, good taste and shortening dissolution time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of Azithromycin Orally Disintegrating Tablets
[0045] Component Weight Weight %
[0046] Azithromycin 100g 33.3%
[0047] Mannitol 88g 29.3%
[0048] Aspartame 3g 1%
[0049] Menthol 1g 0.4%
[0050] Chocolate Powder Flavor 3g 1%
[0051] appropriate amount of water
[0052] Low Substituted Hydroxypropyl Cellulose 18g 6%
[0053] Microcrystalline Cellulose 72g 24%
[0054] Citric acid 3g 1%
[0055] Sodium bicarbonate 9g 3%
[0056] Magnesium Stearate 3g 1%
[0057] Total weight 300g
[0058] A total of 1000 pieces were made
[0059] Concrete preparation method is as follows:
[0060] Weigh azithromycin, mannitol, aspartame, menthol, and chocolate powder flavors that have passed through a 100-mesh sieve, mix them evenly, add an appropriate amount of aqueous solution to granulate, and weigh the additional excipients low-substituted hydroxypropyl cellulose and microcrystalline fiber according to the prescription Sodium glutamate, citric ...
Embodiment 2
[0061] Example 2: Preparation of Azithromycin Orally Disintegrating Tablets
[0062] Component Weight Weight %
[0063] Azithromycin 100g 25%
[0064] Erythrose 125g 31.25%
[0065] Aspartame 4g 1%
[0066] Menthol 1g 0.25%
[0067] Chocolate Powder Flavor 8g 2%
[0068] 50% ethanol appropriate amount
[0069] Low Substituted Hydroxypropyl Cellulose 32g 8%
[0070] Microcrystalline Cellulose 96g 24%
[0071] Citric acid 6g 1.5%
[0072] Sodium bicarbonate 24g 6%
[0073] Magnesium Stearate 4g 1%
[0074] Total weight 400g
[0075] A total of 1000 pieces were made
[0076] Concrete preparation method is as follows:
[0077] Weigh azithromycin, erythrose, aspartame, menthol, and chocolate powder essence that have passed through a 100-mesh sieve, mix well, add an appropriate amount of 50% ethanol solution to granulate, and mix low-substituted hydroxypropyl cellulose, microcrystalline cellulose , citric acid and sodium bicarbonate are mixed evenly with dry granules, a...
Embodiment 3
[0078] Example 3: Preparation of Azithromycin Orally Disintegrating Tablets
[0079] Component Weight Weight %
[0080] Azithromycin 125g 31.25%
[0081] Erythrose 125g 31.25%
[0082] Aspartame 4g 1%
[0083] Menthol 2g 0.5%
[0084] Chocolate Powder Flavor 8g 2%
[0085] 50% ethanol appropriate amount
[0086] Crospovidone 40g 10%
[0087] Microcrystalline Cellulose 80g 20%
[0088] Citric acid 8g 2%
[0089] Sodium bicarbonate 4g 1%
[0090] Magnesium Stearate 4g 1%
[0091] Total weight 400g
[0092] A total of 1000 pieces were made
[0093] Concrete preparation method is as follows:
[0094] Weigh azithromycin, erythrose, aspartame, menthol, and chocolate powder essence that have passed through a 100-mesh sieve, mix well, add an appropriate amount of 50% ethanol to granulate, and mix crospovidone, microcrystalline cellulose, and citric acid And sodium bicarbonate and dry granules are mixed evenly, and magnesium stearate is added after the semi-finished produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com