Health-care product for treating cardiovascular and cerebralvascular disease
A technology for cardiovascular and cerebrovascular diseases and health care products, applied in cardiovascular system diseases, blood diseases, extracellular fluid diseases, etc., can solve the problems of large side effects and single effect, and achieve increased content, increased activity, and protection of brain and cardiomyocytes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
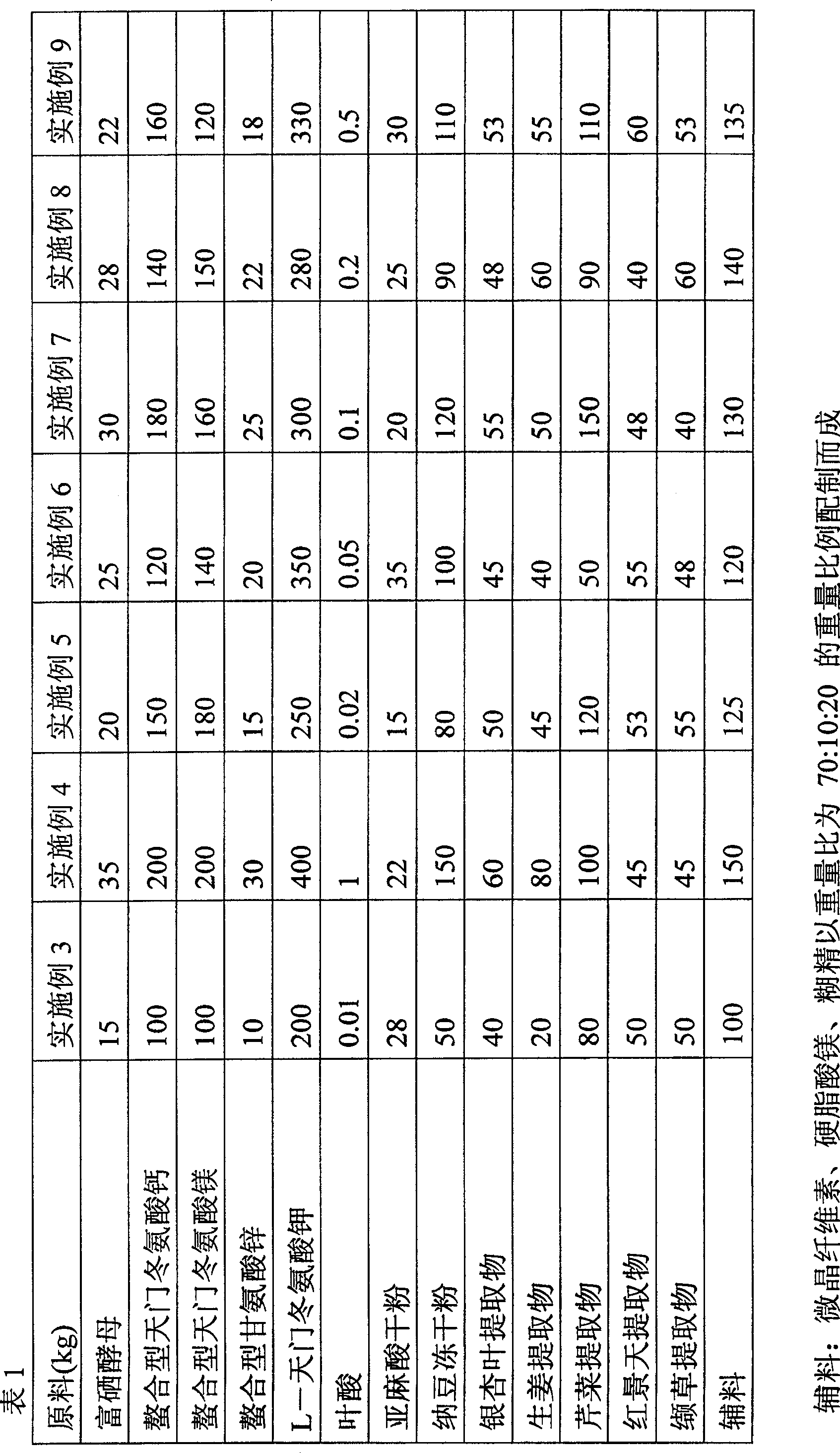
[0084] 25 tons of selenium-enriched yeast; 150 tons of chelated calcium aspartate; 150 tons of chelated magnesium aspartate; 20 tons of chelated zinc glycinate; 300 tons of L-potassium aspartate; 0.15 tons of folic acid; 25 tons of dry powder of linolenic acid; 90 tons of freeze-dried natto powder; 50 tons of ginkgo leaf extract; 50 tons of ginger extract; 100 tons of celery extract; 50 tons of rhodiola extract; 50 tons of valerian extract;
[0085] Adjuvant materials can also be added to the above-mentioned raw materials to facilitate the preparation of tablets. Excipients: microcrystalline cellulose, magnesium stearate, dextrin totaling 130 tons.
[0086] 1. Crush the above raw materials and auxiliary materials to 80-200 mesh;
[0087] 2. Put the above raw materials and auxiliary materials together in the mixing tank and mix them thoroughly;
[0088] 3. Feed the material in the tablet press and press the tablet;
Embodiment 2
[0090] Selenium-enriched yeast 25mg (containing 50 micrograms of selenium); chelated calcium aspartate (containing 10% calcium) 150mg (containing Ca: 15mg); chelated magnesium aspartate (containing 10% magnesium) 150mg (contains Mg: 15mg); chelated zinc glycinate (contains 20% zinc) 20mg (contains Zn: 4mg); L-potassium aspartate (contains about 16% potassium) 300mg (contains K: 50mg); Folic acid 0.1mg (100μg); Linolenic acid dry powder 25mg (containing linolenic acid: 20mg); Natto freeze-dried powder 100mg; Ginkgo biloba extract 50mg; Ginger extract 50mg; Celery extract 100mg; Rhodiola rosea extract 50mg; Grass extract 50 mg; excipients: microcrystalline cellulose, magnesium stearate, and dextrin prepared at a weight ratio of 70:10:20, totaling 130 mg. Prepared according to the method described in Example 1.
Embodiment 3-9
[0092] Examples 3-9 were prepared according to the method described in Example 1, and the proportions are shown in Table 1.
[0093]
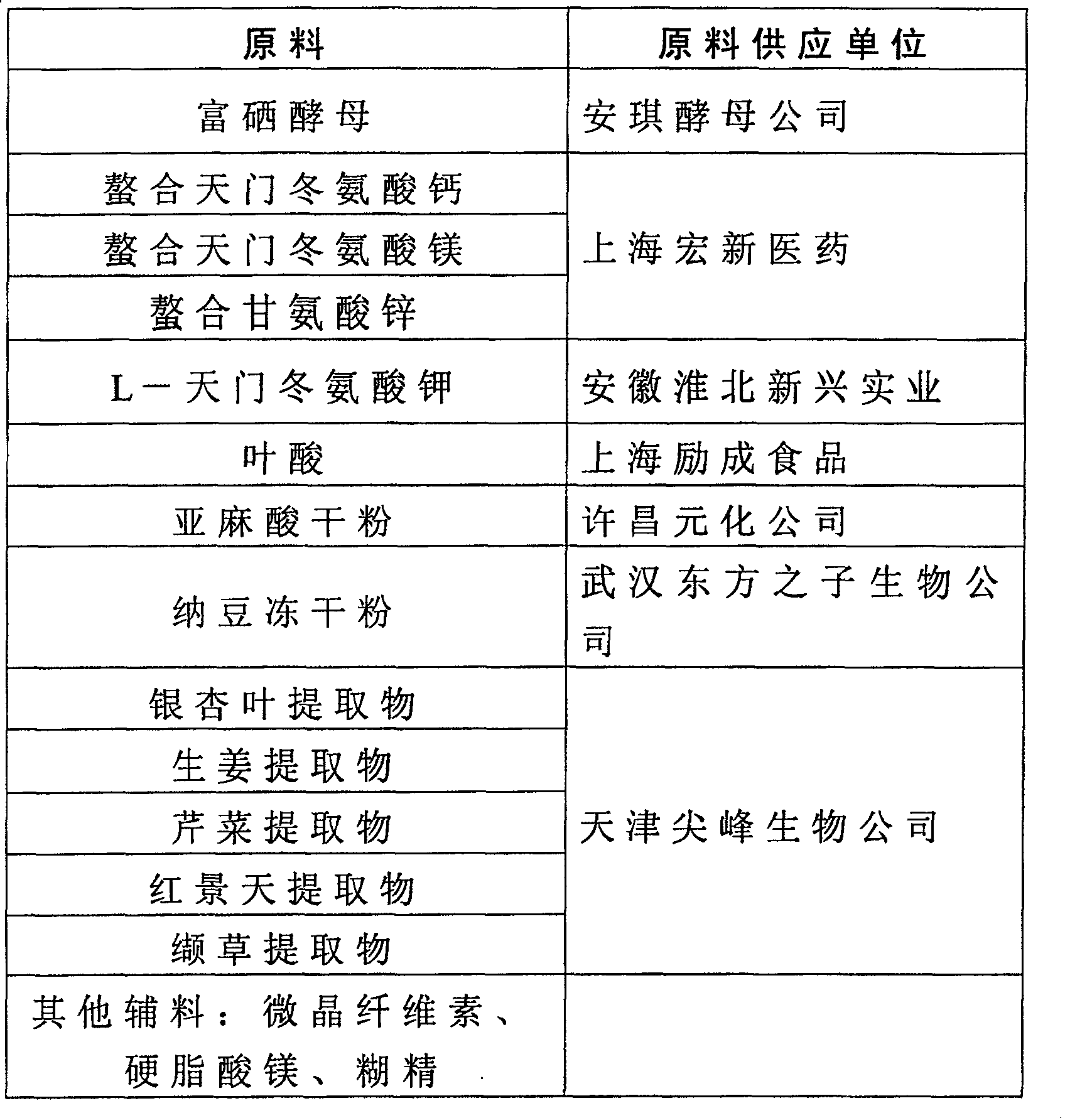
[0094] The manufacturer of raw materials used in the foregoing examples:
[0095] Table 2
[0096]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com