Process for preparing comparison solution for measuring polymeric substance in Cefepime, its salt raw material and preparation
A technology of reference substance solution and cefepime, which is applied in measuring devices, material separation, and analysis of materials, can solve problems such as poor reproducibility, small association peaks, and large association peaks to achieve reproducibility Good, stable peak area, and stable associations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Chromatographic conditions and system suitability test
[0031] Sephadex G-10 (40-120 μm) is used as filler, the inner diameter of the glass column is 1.3-1.8 cm, and the bed volume is 50-200 ml. Mobile phase A is 0.01mol / L phosphate buffer containing 3.5% ammonium sulfate (take disodium hydrogen phosphate 2.19g and sodium dihydrogen phosphate 0.54g, add water 1000ml to dissolve, adjust the pH value to 7.0), mobile phase B is 0.01% sodium lauryl sulfate solution; the flow rate is 1ml per minute; the detection wavelength is 254nm. Use mobile phase A as the mobile phase, inject 200 μl of 1 mg / ml blue dextran 2000 solution into the sample for determination, the number of theoretical plates should not be less than 900, the tailing factor should be 0.75-1.5, and the reference solution should be mobile phase B For the mobile phase, repeated injection of 200μl, the relative deviation of the peak area value should be less than 5.0%.
[0032] Preparation of reference substance...
Embodiment 2
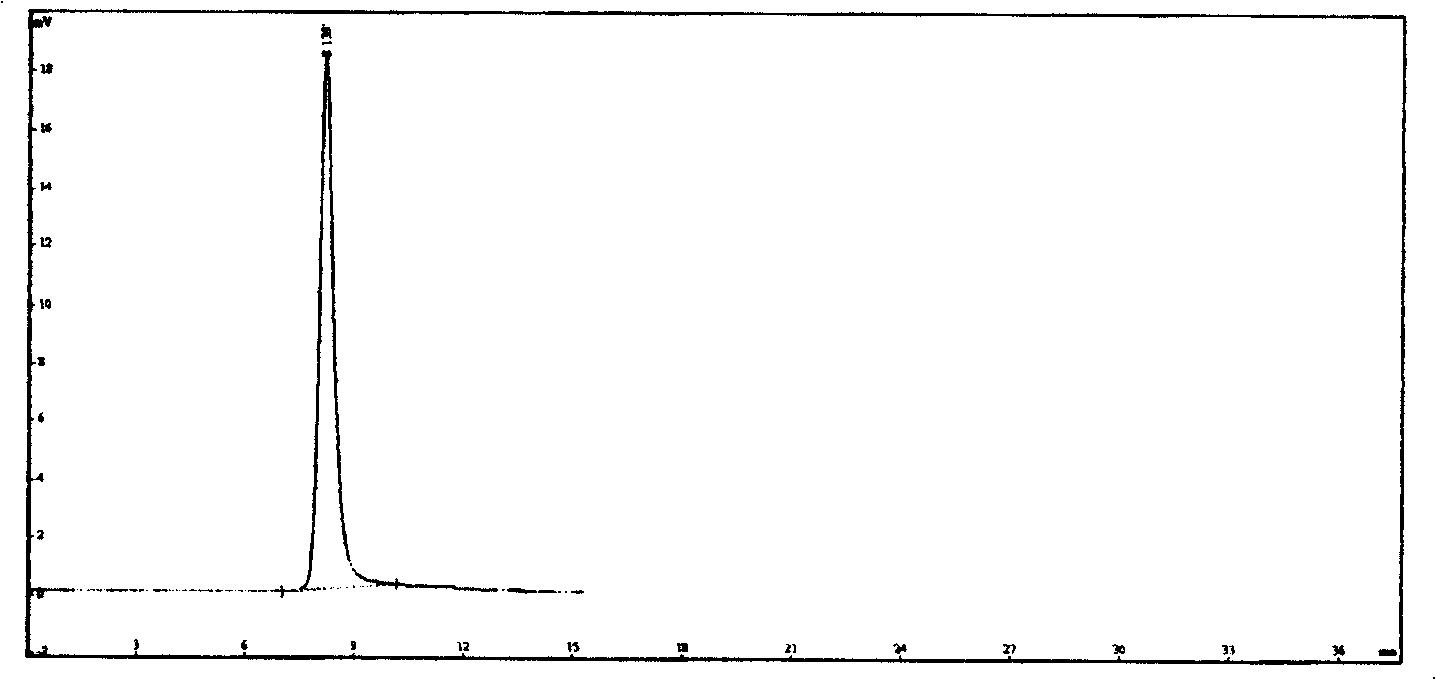
[0035] Same as in Example 1, the preparation method of the reference substance solution: accurately weigh 5 mg of the cefepime reference substance, add borax-sodium carbonate buffer solution (pH=11) to dissolve and dilute to 50 ml, shake well, take a water bath at 40° C. for 1 hour, and cool to room temperature, sample injection and determination of the resulting spectrum as figure 2 .
Embodiment 3
[0037] With embodiment 1, only the preparation of reference substance solution is the preparation of reference substance solution: accurately weigh 5 mg of cefepime reference substance, add 40 ml of water to dissolve, adjust pH value to 11.5 with 0.05N sodium carbonate solution, dilute to 50 ml with water, Shake well, leave at room temperature for 8 hours, and set aside. The chromatogram obtained by the injection measurement is as follows image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com