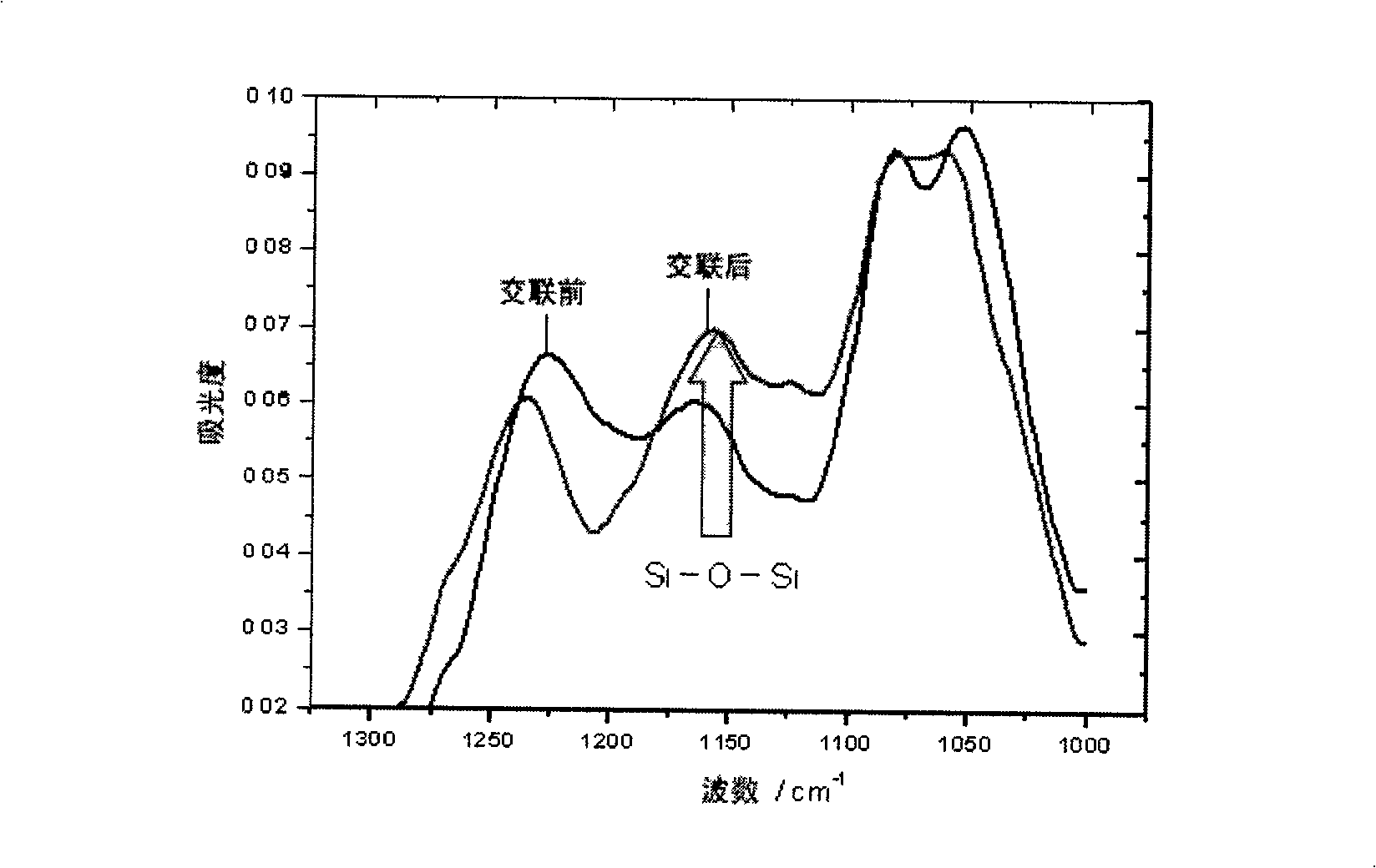
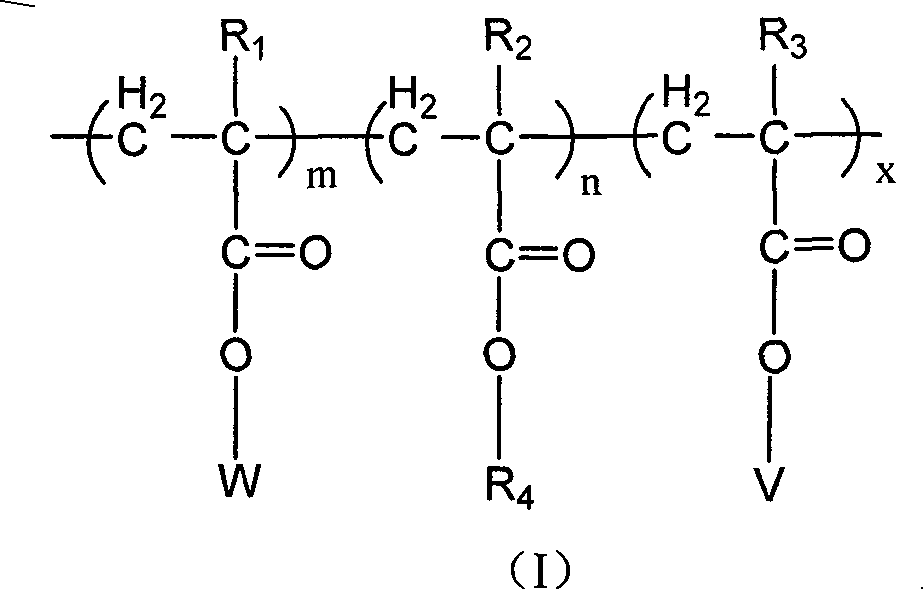
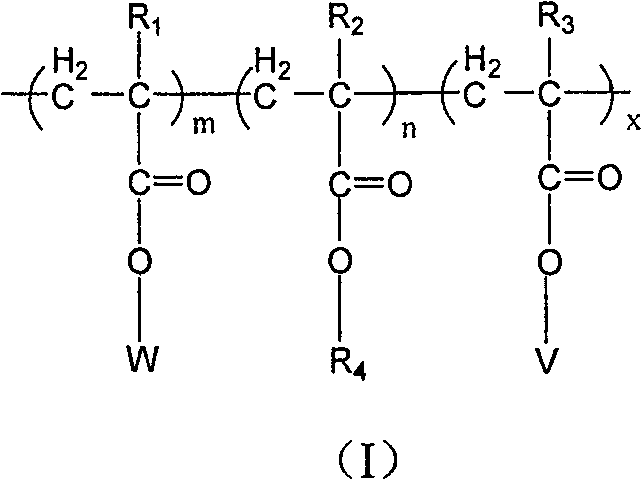
Copolymer of imitating structure of cell membrane, prepartion method and application thereof
A technology of imitating cell outer layer membrane and membrane structure, applied in the field of cross-linkable acrylate terpolymer, can solve the problem of unstable tetrapolymer of amphiphilic polymer coating and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Get methacryloxypropyl trimethoxy silicon (MPS) monomer 0.58g, n-octadecyl methacrylate (SMA) monomer 3.3g, dissolve in 50ml isopropanol in a 250ml three-necked bottle : THF=3:1 (V / V) in anhydrous mixed solvent. 3.0 g of methacryloyloxyethoxyphosphorylcholine (MPC) and 0.020 g of initiator AIBN were respectively dissolved in 40 ml of the above mixed solvent. After 30 minutes of deoxygenation by nitrogen gas, 30% of the MPC solution and AIBN solution were added dropwise at 70°C at the same time, and the remaining part was added dropwise within 3 hours. Continue to reflux condensation at 70°C and stir for 10 hours. The product was precipitated with ether, rinsed, and washed with acetonitrile. use after drying 1 The content of each component was determined by H NMR spectrum. In the silane group (Si-C H 2 -) The chemical shift of the proton is ~0.7ppm; the chemical shift of the proton in the long chain alkyl group is 1.3ppm; the chemical shift of the proton in the pho...
Embodiment 2
[0037] Take 12g of methacryloxypropyltrimethoxysilane (MPS) monomer and 4.2g of n-butyl methacrylate (BMA) monomer and dissolve them in 80ml of absolute ethanol in a 250ml three-necked bottle. 3.0 g of methacryloyloxyethoxyphosphorylcholine (MPC) and 0.030 g of initiator AIBN were respectively dissolved in 40 ml of absolute ethanol. After passing through nitrogen to remove oxygen for 30 minutes, at 60° C., 30% of the MPC solution and AIBN solution were added dropwise at the same time, and the remaining part was added dropwise within 2 hours. It was then condensed under reflux at 65°C and stirred for 16 hours. The product was precipitated with ether, rinsed, washed with acetonitrile, and dried with 1 The molar content of each component of MPC, BMA and MPS measured by H nuclear magnetic resonance spectrum is 21%, 67% and 12%.
Embodiment 3
[0039] Get methacryloxypropyltrimethoxysilane (MPS) monomer 0.90g and dodecyl methacrylate (DMA) monomer 2.6g in the ethanol that dissolves in 80ml in the 250ml three-necked bottle: tetrahydrofuran=4 : 1 in an anhydrous mixed solvent. 3.5 g of methacryloyloxyethyltrimethylammonium chloride (DMC) and 0.030 g of initiator AIBN were respectively dissolved in 30 ml of a mixed solvent of ethanol:tetrahydrofuran=2:1. After passing nitrogen gas to remove oxygen for 30 minutes, 30% of the DMC and AIBN solution was added dropwise at 65° C., and the remaining part was added dropwise within 2 hours. It was then reflux condensed at 65°C and stirred for 24 hours. The product was precipitated with ether, rinsed, washed with acetonitrile, and dried with 1 The molar content of each component of DMC, DMA and MPS measured by H nuclear magnetic resonance spectrum is 60%, 29% and 11%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com