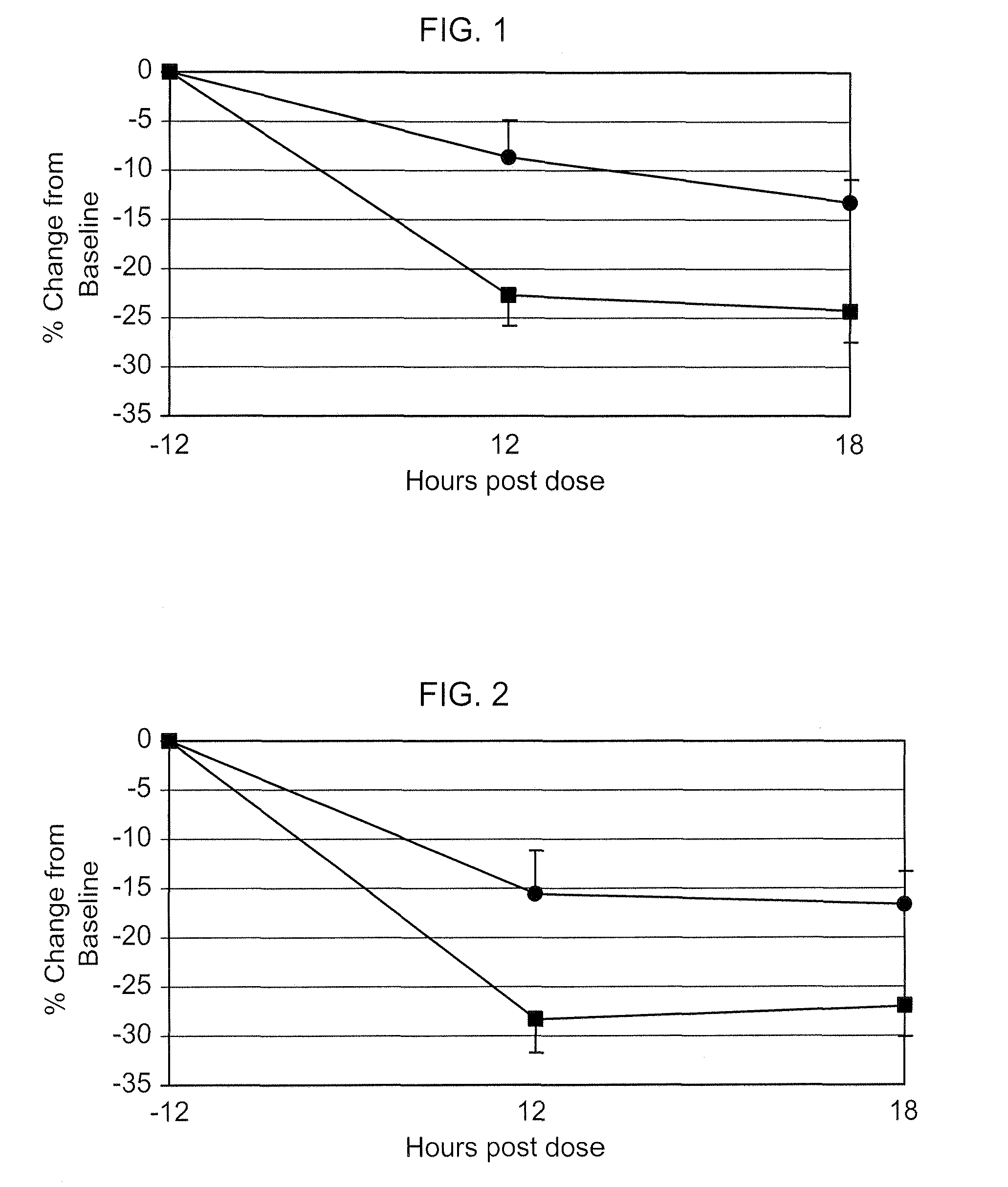
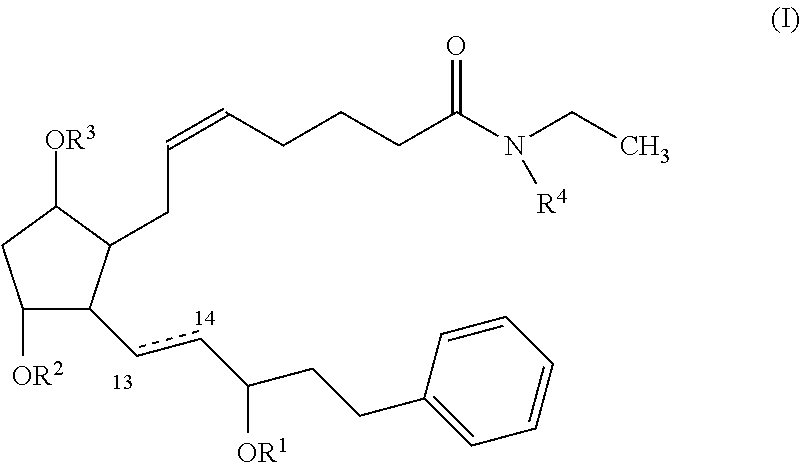
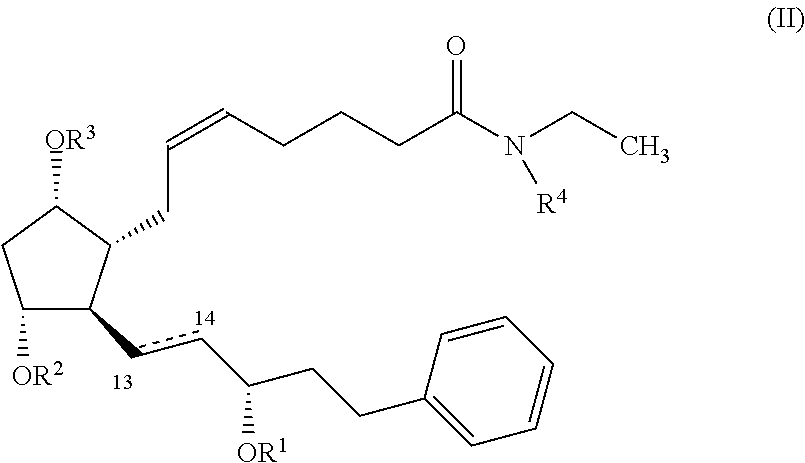
Nitric oxide donating prostamides
a prostamide and nitric oxide technology, applied in the field of prostaglandin derivatives, can solve the problems of eye pressure increasing to unhealthy levels, affecting the effect of eye pressure, so as to reduce the level, maintain the pressure, and reduce the eye pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0130]In the examples described below, unless otherwise indicated, all temperatures are set forth in degrees Celsius and all parts and percentages are by weight. Reagents may be purchased from commercial suppliers, such as Sigma-Aldrich Chemical Company, Acros Organics, or Lancaster Synthesis Ltd. and may be used without further purification unless otherwise indicated. Tetrahydrofuran (THF), methylene chloride (CH2Cl2 or DCM), N,N-dimethylacetamide (DMA), acetonitrile (MeCN or ACN), and N,N-dimethylformamide (DMF) may be purchased from Aldrich in Sure-Seal bottles and used as received. All solvents may be purified using standard methods known to those skilled in the art, unless otherwise indicated. Diethyl ether is abbreviated as Et2O. Ethyl acetate is abbreviated as EtOAc or EA. Trifluoroacetic acid is abbreviated as TFA. Acetic acid is abbreviated as HOAc or AcOH. Trifluoromethanesulfonate, or triflate, is abbreviated as “OTf.” tert-Butoxycarbonyl is abbreviated as BOC. 4-(N,N-Dim...
example a-1
NCX 469
(1S,2E)-3-{(1R,2R,3S,5R)-2-[(2Z)-7-(Ethylamino)-7-oxohept-2-en-1-yl]-3,5-dihydroxycyclopentyl}-1-(2-phenylethyl)prop-2-en-1-yl 4-(Nitrooxy)butanoate (A-1)
[0137]Following an analogous procedure from Bundy, G. L.; Peterson, D. C.; Cornette, J. C.; Miller, W. L.; Spilman, C. H.; Wilks, J. W. J. Med. Chem. 1983, 26, 1089-1099, to a solution of bimataprost (Cayman Chemicals; 200 mg, 0.481 mmol) in dichloromethane (4.8 mL) was added butylboronic acid (55.0 mg, 0.541 mmol). After 1 h at 42° C., some dichloromethane was evaporated and fresh dichloromethane added. This evaporation-fresh solvent addition sequence was repeated 3 times. 4 Å molecular sieves were added and the mixture stirred at 42° C. for 18 h. 4-Bromobutyryl chloride (0.061 mL; 0.53 mL) was added and allowed to stir at ambient temperature for 48 h. The solvent was removed under reduced pressure and the residue dissolved in acetonitrile (2.4 mL). Silver nitrate (163 mg, 0.962 mmol) was added and allowed to stir at ambien...
example b-1
NCX470
(1S,2E)-3-{(1R,2R,3S,5R)-2-[(2Z)-7-(Ethylamino)-7-oxohept-2-en-1-yl]-3,5-dihydroxycyclopentyl}-1-(2-phenylethyl)prop-2-en-1-yl 6-(Nitrooxy)hexanoate (B-1)
Step 1: (5Z)-7-{(6R,7R)-3-Butyl-7-[(1E,3S)-3-hydroxy-5-phenylpent-1-en-1-yl]-2,4-dioxa-3-borabicyclo[3.2.1]oct-6-yl}-N-ethylhept-5-enamide (b-1)
[0146]
[0147]Following an analogous procedure from Bundy, G. L.; Peterson, D. C.; Cornette, J. C.; Miller, W. L.; Spilman, C. H.; Wilks, J. W. J. Med. Chem. 1983, 26, 1089-1099, to a solution of bimatoprost (Cayman Chemicals 16820, Lot 188757; 679 mg, 1.63 mmol) in DCM (10.9 mL) was added butylboronic acid (187 mg, 1.84 mmol). After 1 hour at 42° C., solvent was removed under reduced pressure and dried under high vacuum pump for 2 hours. Fresh DCM was added and stirred at 42° C. for another hour. Solvent was removed and dried under high vacuum pump for 1.5 hour. Fresh DCM was added again and stirred at 42° C. for 16 hours. Solvent was evaporated and dried in vacuum oven at 45° C. for 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| nm wavelength | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com