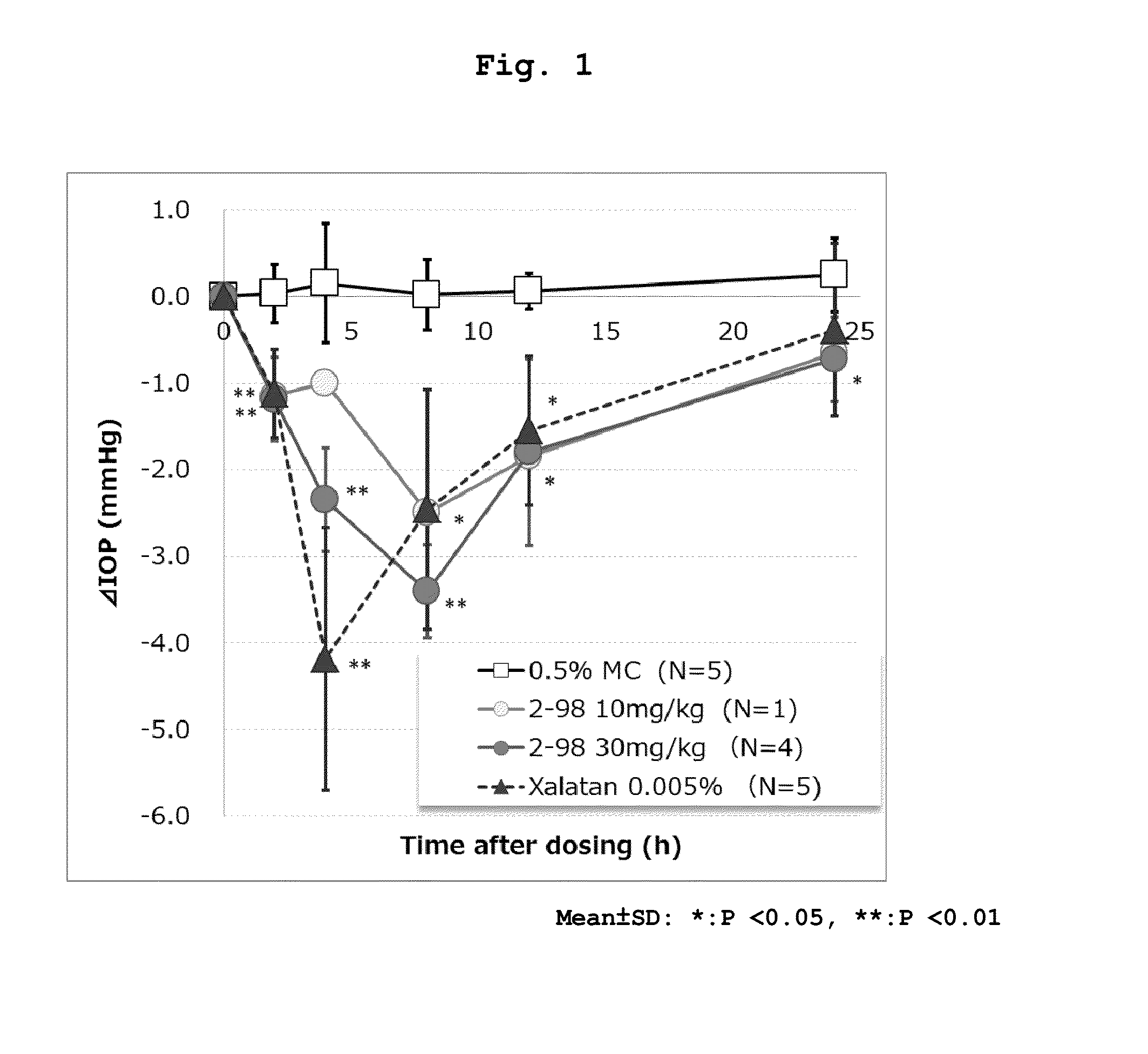
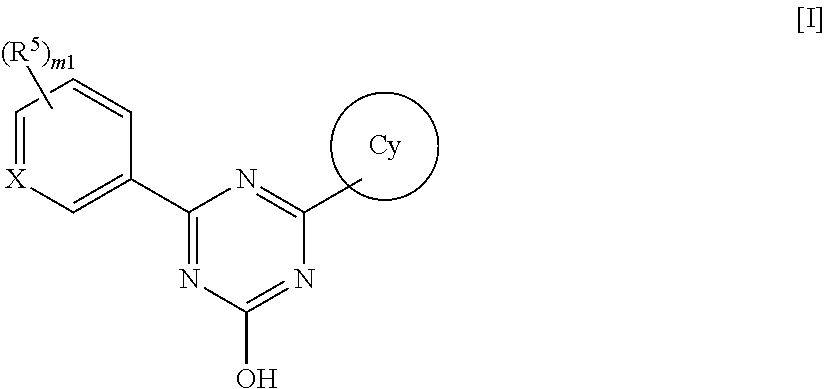
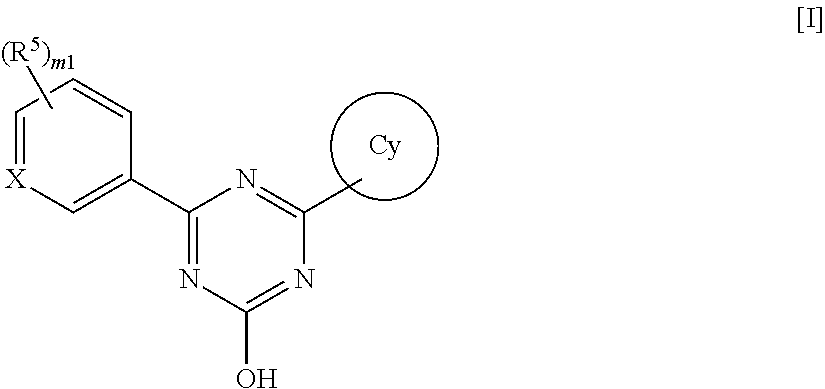
Triazine compounds and pharmaceutical use thereof
a technology of triazine and compound, applied in the field of triazine compound, can solve the problems of side effects of nsaid, increase the risk of stomach perforation, bleeding and the like, and disrupt the balance, and achieve the effect of suppressing pge2 production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
Synthesis of N-(4-chloro-3-{4-[4-(2,2-dimethylpropoxyl)phenyl]-6-hydroxy-1,3,5-triazin-2-yl}benzyl)-3,3,3-trifluoro-2,2-dimethylpropionamide (Example No. 1-86)
[0584]
(1) 2-chloro-4-[4-(2,2-dimethylpropoxyl)phenyl]-6-methoxy-1,3,5-triazine
[0585]
[0586]Under an argon atmosphere, a suspension of 4-(2,2-dimethylpropoxyl)phenylboronic acid (2.0 g, 9.6 mmol), 2,4-dichloro-6-methoxy-1,3,5-triazine (3.5 g, 19 mmol), [1,1′-bis(diphenylphosphino) ferrocene]palladium(II)dichloride dichloromethane adduct (1.1 g, 0.96 mmol) and 2M aqueous sodium carbonate solution (14 ml, 29 mmol) in toluene (20 ml) was stirred at 100° C. for 3.5 hr. At room temperature, to the reaction mixture were added water and ethyl acetate and the mixture was partitioned. The organic layer was washed with saturated aqueous sodium hydrogen carbonate, washed with saturated brine, dried over sodium sulfate, filtered to remove sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column ...
production example 2
Synthesis of 1-[4-chloro-3-(4-hydroxy-6-phenyl-1,3,5-triazin-2-yl)-benzyl]-3,3-dimethyl-1,3-dihydroindol-2-one (Example No. 1-258)
[0603]
(1) 2-(5-bromomethyl-2-chlorophenyl)-4-methoxy-6-phenyl-1,3,5-triazine
[0604]
[0605]By a method similar to that in Production Example 1 (1) and (2), and using 2,4-dichloro-6-methoxy-1,3,5-triazine, 2-chloro-5-hydroxymethylphenylboronic acid, and phenylboronic acid instead of 4-(2,2-dimethylpropoxyl)phenylboronic acid, [4-chloro-3-(4-methoxy-6-phenyl-1,3,5-triazin-2-yl)phenyl]methanol was obtained.
[0606]Under an argon atmosphere, to a solution of the obtained [4-chloro-3-(4-methoxy-6-phenyl-1,3,5-triazin-2-yl)phenyl]methanol (0.47 g, 1.4 mmol) and triphenylphosphine (0.56 g, 2.1 mmol) in chloroform (4.5 ml) was added carbon tetrabromide (0.71 g, 2.1 mmol) under ice-cooling. The reaction mixture was stirred at room temperature for 10 min, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: n-hex...
production example 3
Synthesis of N-[4-chloro-3-(4-hydroxy-6-phenyl-1,3,5-triazin-2-yl)benzyl]-N-ethyl-3,3,3-trifluoro-2,2-dimethylpropionamide (Example No. 1-263)
[0614]
(1) [4-chloro-3-(4-methoxy-6-phenyl-1,3,5-triazin-2-yl)benzyl]ethylamine
[0615]
[0616]Under an argon atmosphere, to 2-(5-bromomethyl-2-chlorophenyl)-4-methoxy-6-phenyl-1,3,5-triazine (0.20 g, 0.51 mmol) obtained in the same manner as in Production Example 2 (1) was added a solution of 2M ethylamine tetrahydrofuran (2.5 ml) at room temperature, and the mixture was stirred for 1 hr. To the reaction mixture were added saturated aqueous sodium hydrogen carbonate and ethyl acetate and the mixture was partitioned. The organic layer was washed with saturated brine, dried over sodium sulfate, filtered to remove sodium sulfate, and concentrated under reduced pressure to give the title compound (0.28 g) as a crude product.
[0617]1H-NMR (400 MHz, CDCl3) δ: 1.14 (3H, t, J=7.2 Hz), 2.70 (2H, q, J=7.2 Hz), 3.86 (2H, s), 4.22 (3H, s), 7.44 (1H, dd, J=8.2,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com