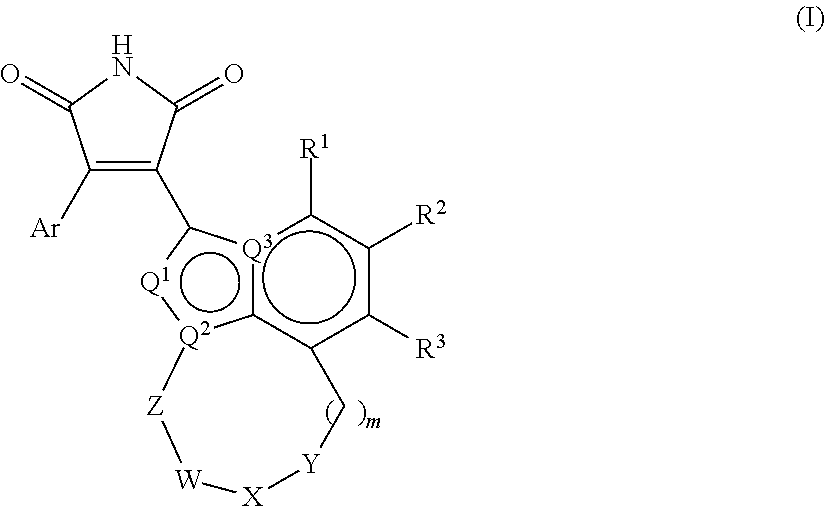
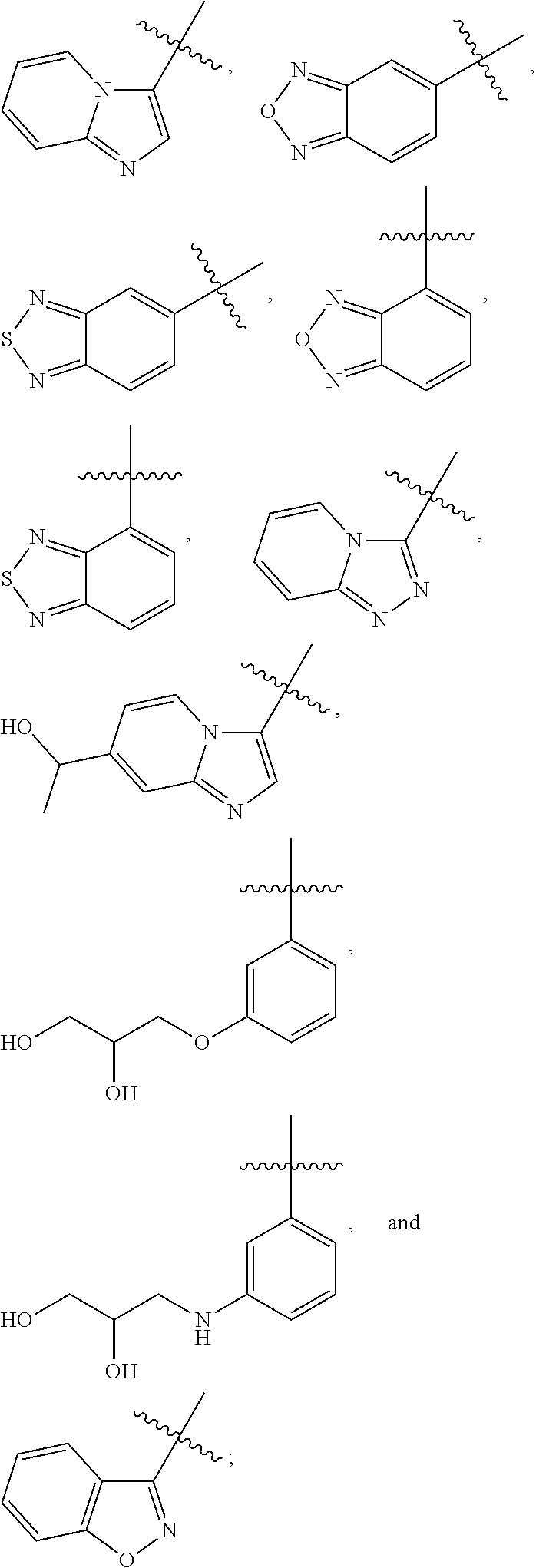
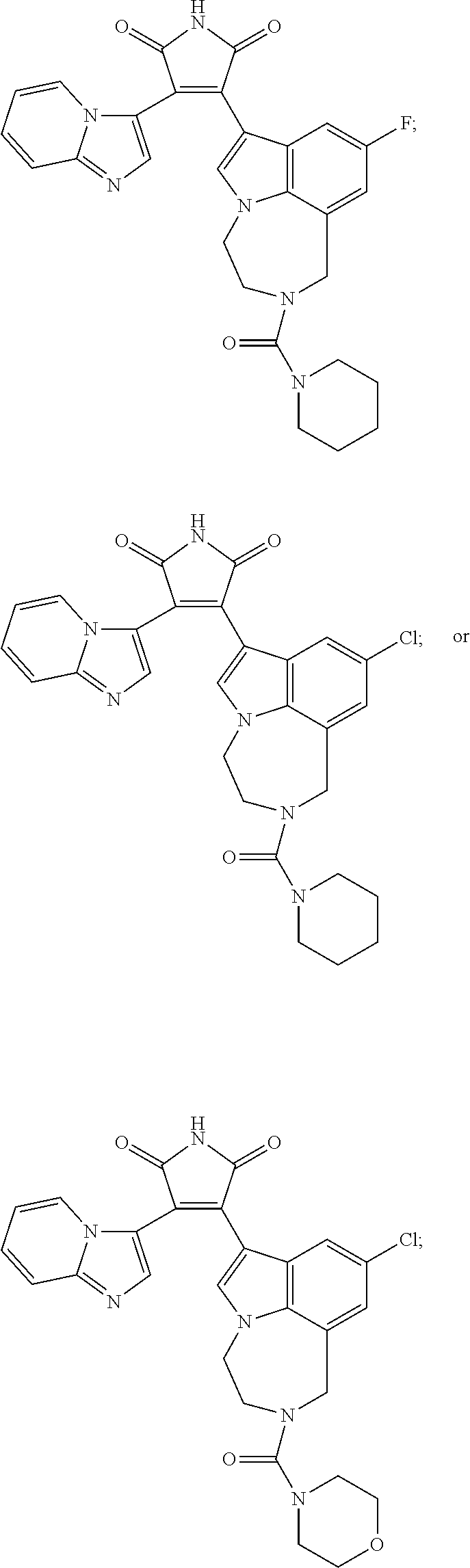
1h-pyrrole-2,5-dione compounds and methods of using them to induce self-renewal of stem/progenitor supporting cells
a technology of pyrrole and pyrrole, which is applied in the field of 1hpyrrole2, 5dione compounds, can solve the problems of increasing hair cell numbers, affecting the survival of stem/progenitor supporting cells, and affecting the survival of stem cells, so as to reduce the activity of tgf beta
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
General Experimental Methods
[0396]1H NMR spectra were recorded on Bruker Avance III 400 MHz and Bruker Fourier 300 MHz and TMS was used as an internal standard.
[0397]LCMS was taken on a quadrupole Mass Spectrometer on Agilent LC / MSD 1200 Series (Column: C18 (50×4.6 mm, 5 μm) operating in ES (+) or (−) ionization mode; T=30° C.
Synthetic Schemes
[0398]
Experimental Procedures:
Synthesis of Intermediate 2.
[0399]
[0400]To a solution of intermediate 1 (20 g, 213 mmol) in MeCN (540 ml) was ethyl (E)-4-oxo-butenoate (28.6 g, 223 mmol). The reaction mixture was heated to 80° C. and stirred for 6 hrs. The reaction mixture was concentrated under reduced pressure, the residue was purified by flash column chromatography (eluted with Dichloromethane / MeOH from 1:0 to 200:1) to give the crude intermediate 2 (25 g) as brown solid.
Synthesis of Intermediate 3.
[0401]
[0402]To a solution of crude intermediate 2 (25 g) in MeOH (100 ml) was added NH3 / MeOH (6 M, 100 ml). The reaction mixture was stirred at roo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| field size | aaaaa | aaaaa |
| field size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com