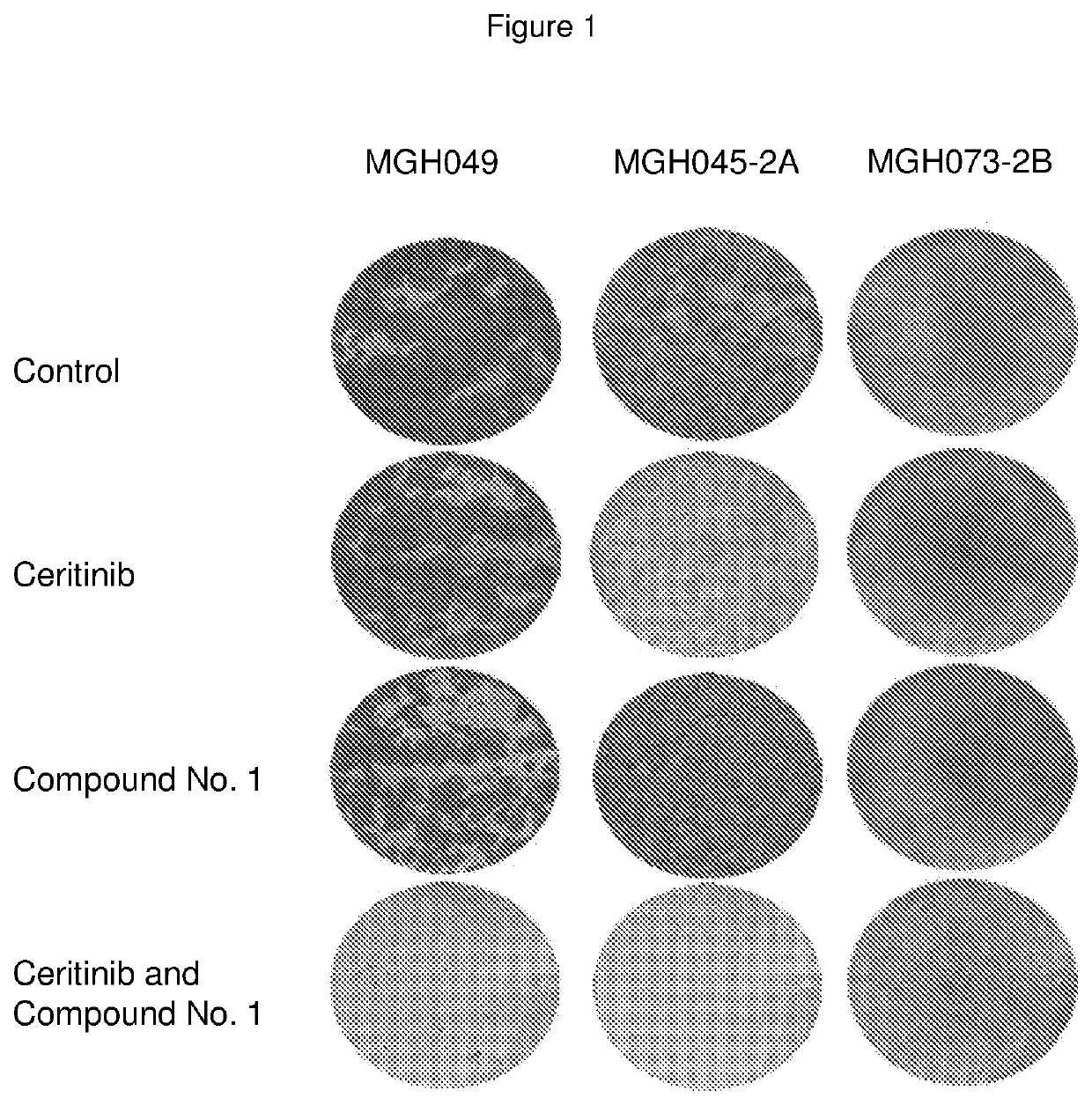
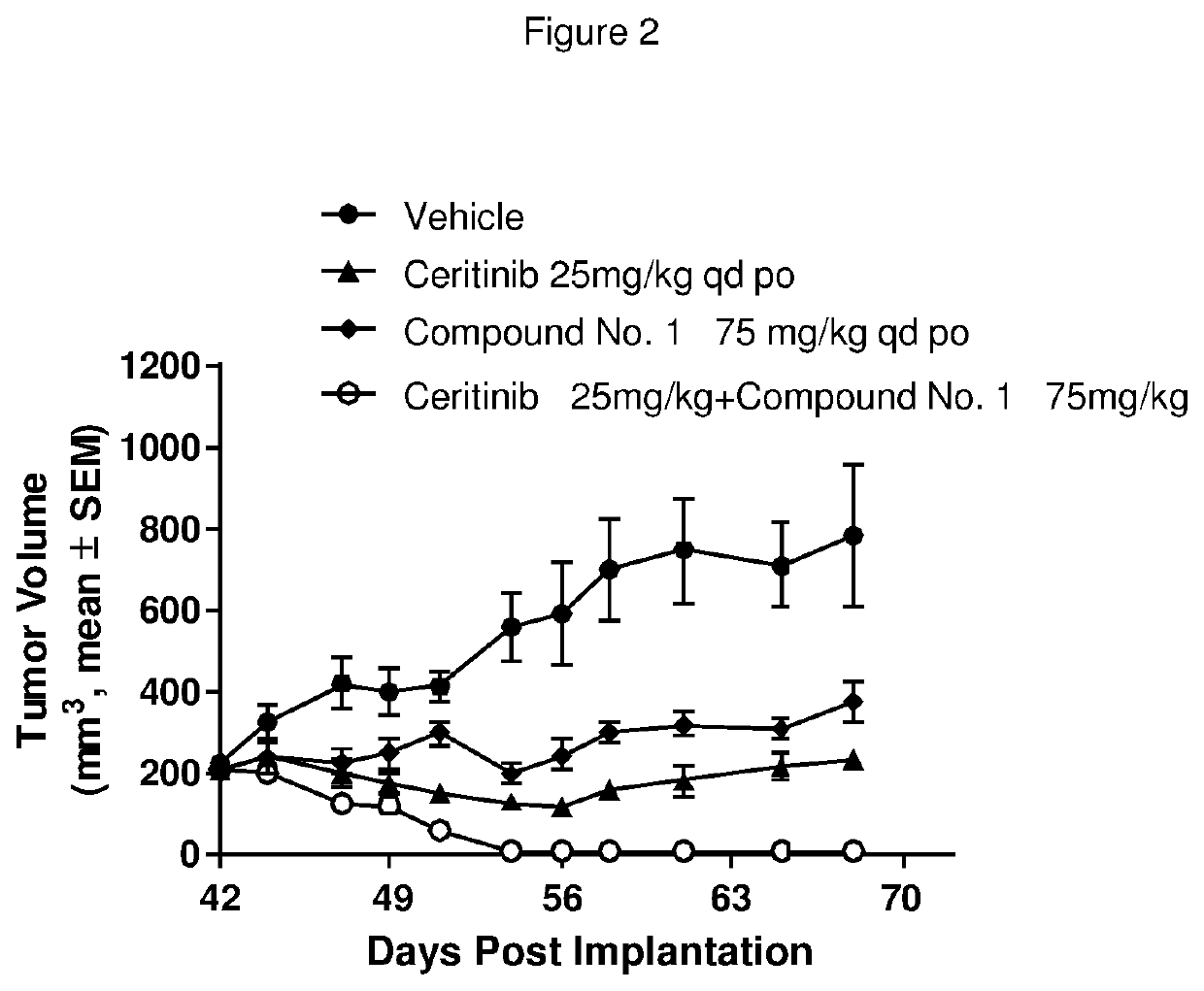
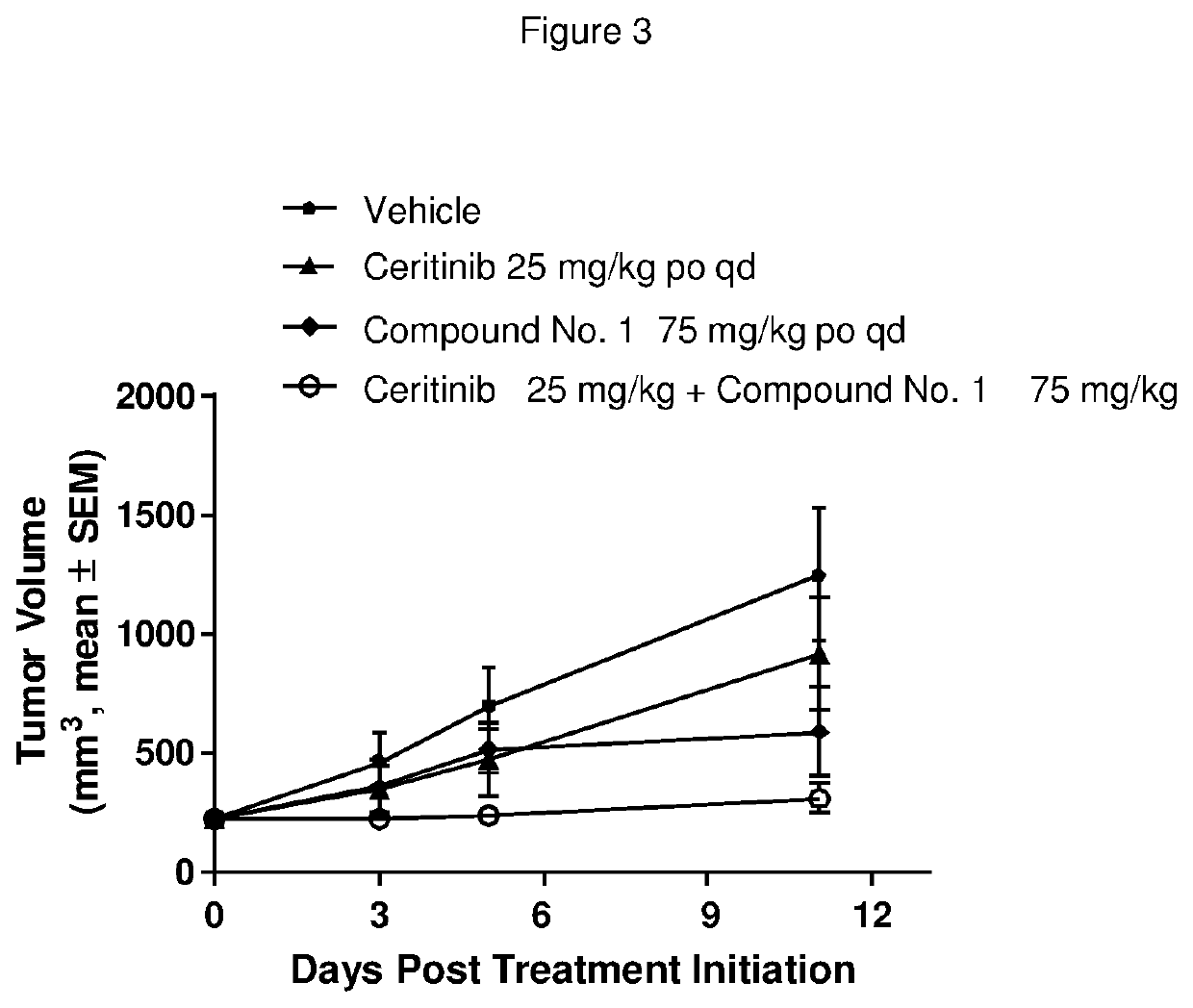
Pharmaceutical combination comprising an alk inhibitor and a shp2 inhibitor
a technology combination drugs, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, organic active ingredients, etc., can solve problems such as resistance and achieve the effect of reducing the ras-gtp loading potential of cells and amplifying the anti-proliferation effect of anaplastic lymphoma kinas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
[0188]A pharmaceutical combination comprising:[0189](i) an ALK inhibitor, or a pharmaceutically acceptable salt thereof, selected from 5-chloro-N2-[2-isopropoxy-5-methyl-4-(4-piperidinyl)phenyl]-N4-[2-(isopropylsulfonyl)phenyl]-2,4-pyrimidinediamine (ceritinib), 9-Ethyl-6,6-dimethyl-8-[4-(4-morpholinyl)-1-piperidinyl]-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (alectinib) and (10R)-7-Amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,1]-benzoxadiazacyclotetradecine-3-carbonitrile (loratinib), and[0190](ii) a SHP2 inhibitor, or a pharmaceutically acceptable salt thereof.
embodiment 2
[0191]A pharmaceutical combination comprising:[0192](i) an ALK inhibitor, or a pharmaceutically acceptable salt thereof, selected from 5-chloro-N2-[2-isopropoxy-5-methyl-4-(4-piperidinyl)phenyl]-N4-[2-(isopropylsulfonyl)phenyl]-2,4-pyrimidinediamine (ceritinib), 9-Ethyl-6,6-dimethyl-8-[4-(4-morpholinyl)-1-piperidinyl]-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile (alectinib) and (10R)-7-Amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,1]-benzoxadiazacyclotetradecine-3-carbonitrile (loratinib), and[0193](ii) a SHP2 inhibitor of Table 1, or a pharmaceutically acceptable salt thereof,
TABLE 1Com-poundNo.StructureName16-(4-amino-4-methylpiperidin- 1-yl)-3-(2,3- dichlorophenyl)pyrazin-2- amine26-(4-aminomethyl)-4- phenylpiperidin-1-yl)-3-(2,3- dichlorophenyl)pyrazin-2- amine36-(4-(aminomethyl)-4- methylpiperidin-1-yl)-3-(2,3- dichlorophenyl)pyrazin-2- amine46-(4-(aminomethyl)-4- methylpiperidin-1-yl)-3-((2- (trifluoromethyl)pyri...
embodiment 3
[0194]The pharmaceutical combination of Embodiment 1 or Embodiment 2, wherein the ALK inhibitor is 5-chloro-N2-[2-isopropoxy-5-methyl-4-(4-piperidinyl)phenyl]-N4-[2-(isopropylsulfonyl)phenyl]-2,4-pyrimidinediamine (ceritinib), or a pharmaceutically acceptable salt thereof.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com