Thermal Energy storage system with enhanced transition array
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Example
Example 1
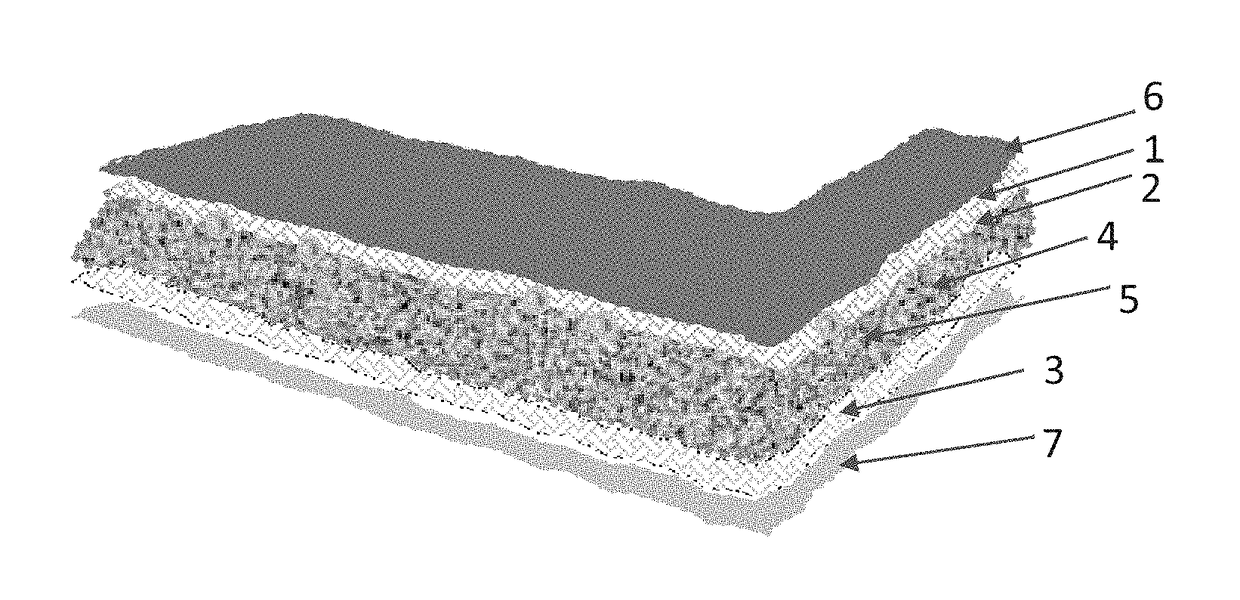
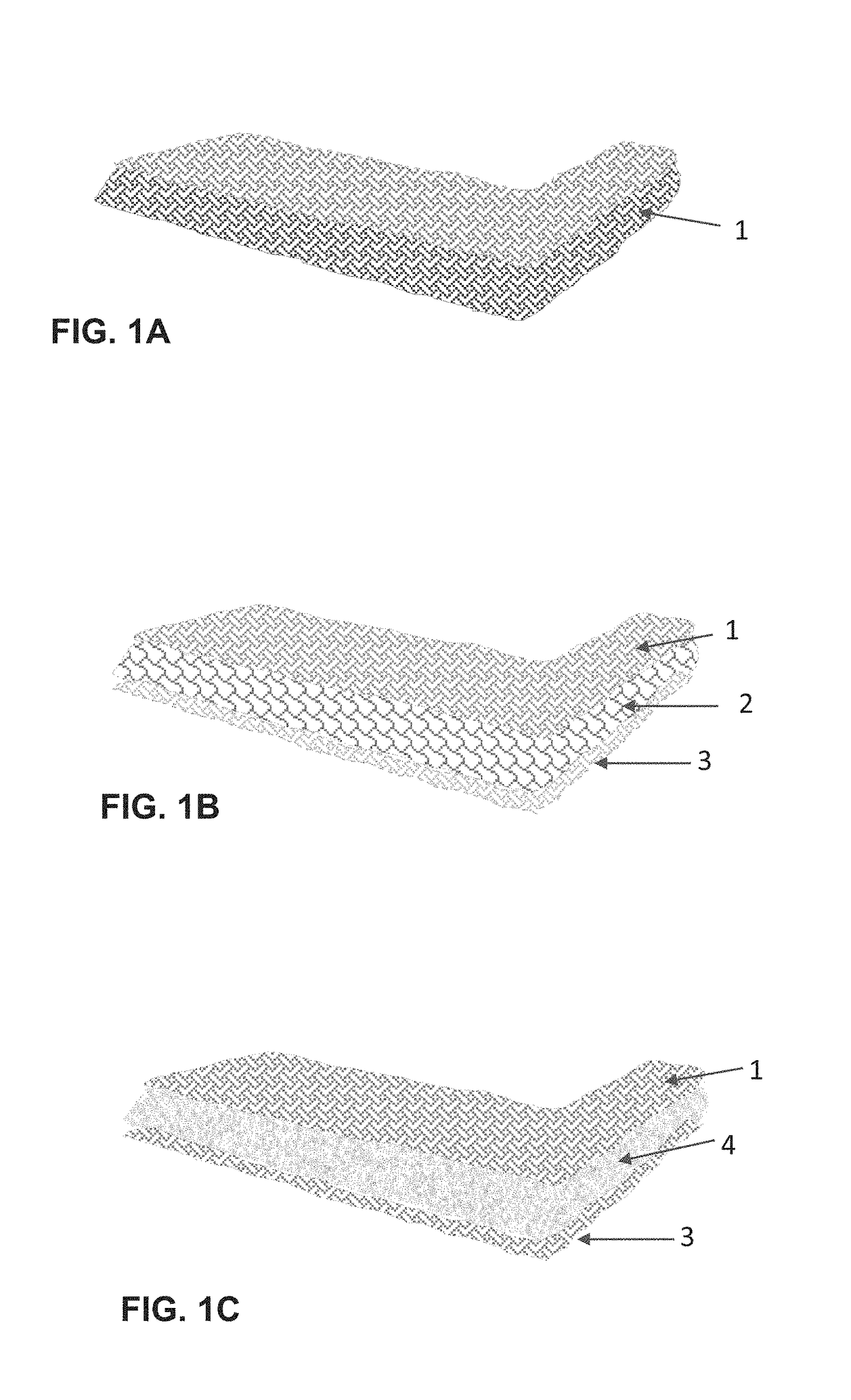
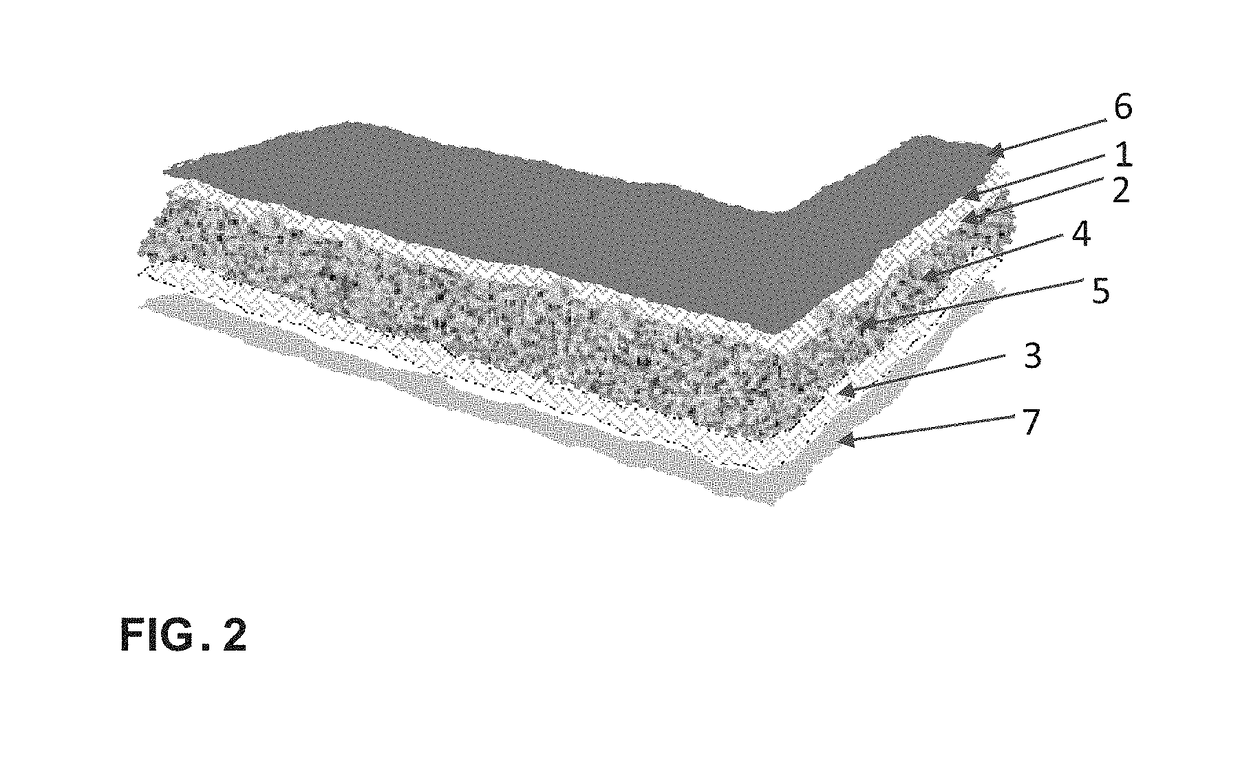
[0030]This example illustrates the 3-D media matrix and fiber network capable of absorbing aqueous and non-aqueous materials. The continuous media and / or its components is modified with crystal modifiers selected from Al, Cu, Fe, FeO, CuO, Cu2O, ZnO, SrO, Al2O3, Fe2O3, SiO2, BaO, NaCl, KCl, LiCl, StCl, Na2CO3, MgCl2, ZnCl2, FeCl3, BaCl2, MgSO4, CuSO4, BaSO4, CaCO3, Na2SO4, Na2B4O7, Sr3(PO4)2, CaB4O7, Na5P3O10, BaS2O3, BaCO3, SrCO3, carbon fibers, carbon black, Magnesium stearate, bentonite, ethylene oxide, propylene oxide, or mixtures thereof in amounts of less than 30 percent by weight. The thermal storage PCM used with the media matrix is selected from inorganic salts and crystalline hydrates for example and not limited to calcium chloride hexahydrate, sodium carbonate dodecahydrate, eutectic mixtures, fatty acids / esters, alcohols, organic eutectics, aliphatic hydrocarbons and combinations thereof.
[0031]Compositions are loaded into the media's components or admixed with t...
Example
Example 2
[0032]This example illustrates the preparation of a salt hydrate PCM composition and loading of the media support. A feed stock calcium chloride is dissolved in an aqueous solvent at moderate temperatures (under 40° C.) to allow a much higher concentration to be achieved. The concentrated brine solution is stripped in purification steps such as filtration or settled particle separation while at elevated temperatures. The concentrated brine solution is cooled to less than 20° C. to crystalize calcium chloride hexahydrate crystals. The resulting crystals are separated and placed in a closed temperature controlled dissolution vessel. The temperature is set to 35-37° C. for the crystals to melt and the temperature is maintained at this level while stirring. The vessel is kept close to prevent water loss. Association of alkali metals made up of Group 1 and group 2 are added to the melted crystals at a total percent weight not to exceed 10%. In one embodiment of a formula, calciu...
Example
Example 3
[0033]A saturated solution of calcium chloride is prepared in an aqueous solvent at 40 C while stirring. The concentrated brine solution is purified to produce the mother liquor. The concentrated brine solution is cooled to less than 20° C. to crystalize calcium chloride hexahydrate crystals. The resulting crystals are separated and placed in a closed temperature controlled dissolution vessel and heated to 35-37° C. An alkali metal salt from group 1 was added to the mix while stirring in amounts of less than 5 percent weight. A 2 percent weight magnesium chloride and 2 percent weight strontium chloride are added to the calcium chloride mixture. Barium sulfate and Titanium oxide are added while stirring in less than 1 percent weight. The formula is added to the media matrix and allowed to be absorbed and distributed in the support media. The loaded media matrix is then placed in a sealed moisture barrier film preferably with aluminum film component to produce the thermal ene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com