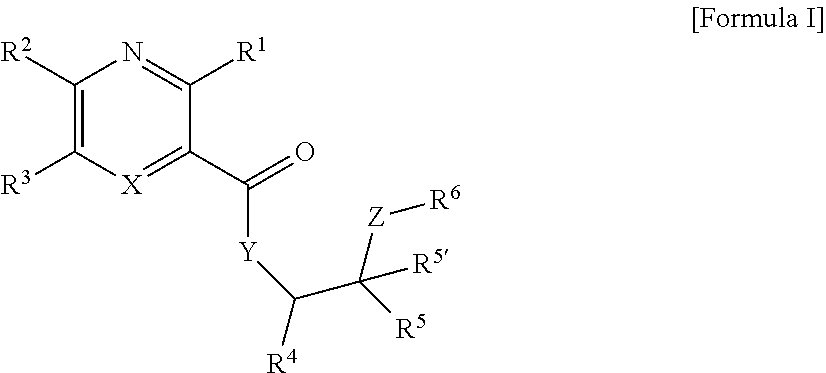
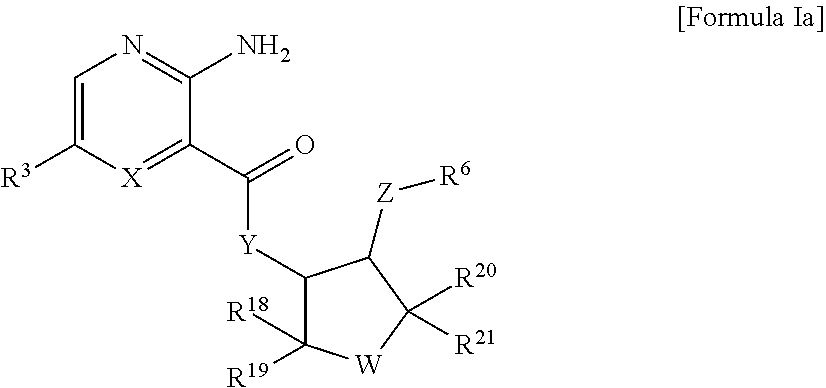
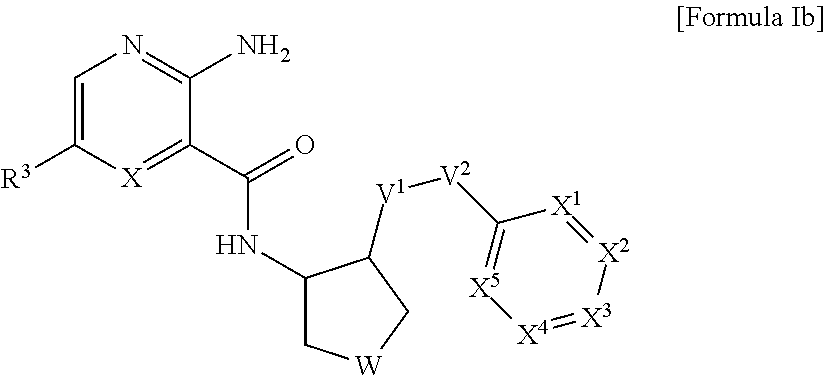
Heteroaryl compounds and their use as therapeutic drugs
a technology of heteroaryl compounds and therapeutic drugs, applied in the field of heteroaryl compounds, can solve the problems of limited potency and lack of selectivity of inhibitors, and it is difficult to find inhibitors that are specific for mer,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
2-amino-N-((1S,2S)-2-(benzyloxy)cyclopentyl)-5-(1-methyl-1H-pyrazol-4-yl)nicotinamide
[0117]To a mixture of intermediate 2 (43 mg, 0.2 mmol) and triethylamine (24 mg, 0.24 mmol) in 2 ml of DMF was added HATU (91 mg, 0.24 mmol) followed by (1S,2S)-2-(benzyloxy)cyclopentan-1-amine (38 mg, 0.2 mmol). The mixture was stirred at room temperature for 1 hr and then saturated sodium bicarbonate solution was added. The mixture was extracted with EtOAc, washed with brine, dried over MgSO4, and concentrated in vacuo. The crude residue was purified by preparative HPLC to afford 46 mg of the title compound.
[0118]1H NMR (600 MHz, CD3OD) δ ppm 1.57-1.69 (m, 1H) 1.72-1.86 (m, 3H) 1.90-2.08 (m, 1H) 2.11-2.21 (m, 1H) 3.93 (s, 3H) 3.96 (dt, J=6.75, 4.26 Hz, 1H) 4.39 (td, J=7.34, 4.11 Hz, 1H) 4.61 (s, 2H) 7.13-7.24 (m, 1H) 7.27 (t, J=7.46 Hz, 2H) 7.32 (d, J=7.04 Hz, 2H) 7.79-7.90 (m, 1H) 8.00 (s, 1H) 8.23 (d, J=1.76 Hz, 1H) 8.46 (d, J=2.35 Hz, 1H);
[0119]MS (ESI, m / z): 392.2 [M+H]+
example 2
2-amino-N-(1R,2R)-2-(benzyloxy)cyclopentyl)-5-(1-methyl-1-pyrazol-4-yl)nicotinamide
[0120]Using (1R,2R)-2-(benzyloxy)cyclopentan-1-amine, the title compound was obtained as described for the example 1.
[0121]MS (ESI, m / z): 392.2 [M+H]+
example 3
2-amino-N-(trans-2-(benzyloxy)cyclopentyl)-5-(1-methyl-1H-pyrazol-4-yl)nicotinamide
[0122]Using trans-2-(benzyloxy)cyclopentan-1-amine, the title compound was obtained as described for the example 1.
[0123]MS (ESI, m / z): 392.2 [M+H]+
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com