Method of Manufacturing a Medical Device
a manufacturing method and technology for medical devices, applied in the field of manufacturing medical devices, can solve the problems of compromising sterility, prone to neglection of precautionary measures, and only stable pharmaceutical drugs adapted for parenteral administration, so as to eliminate or reduce at least one drawback
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0072]When in the following relative expressions, such as “upwards” and “downwards”, are used, these refer to the appended figures and not necessarily to an actual situation of use. The shown figures are schematic representations for which reason the configuration of the different structures as well as their relative dimensions are intended to serve illustrative purposes only.
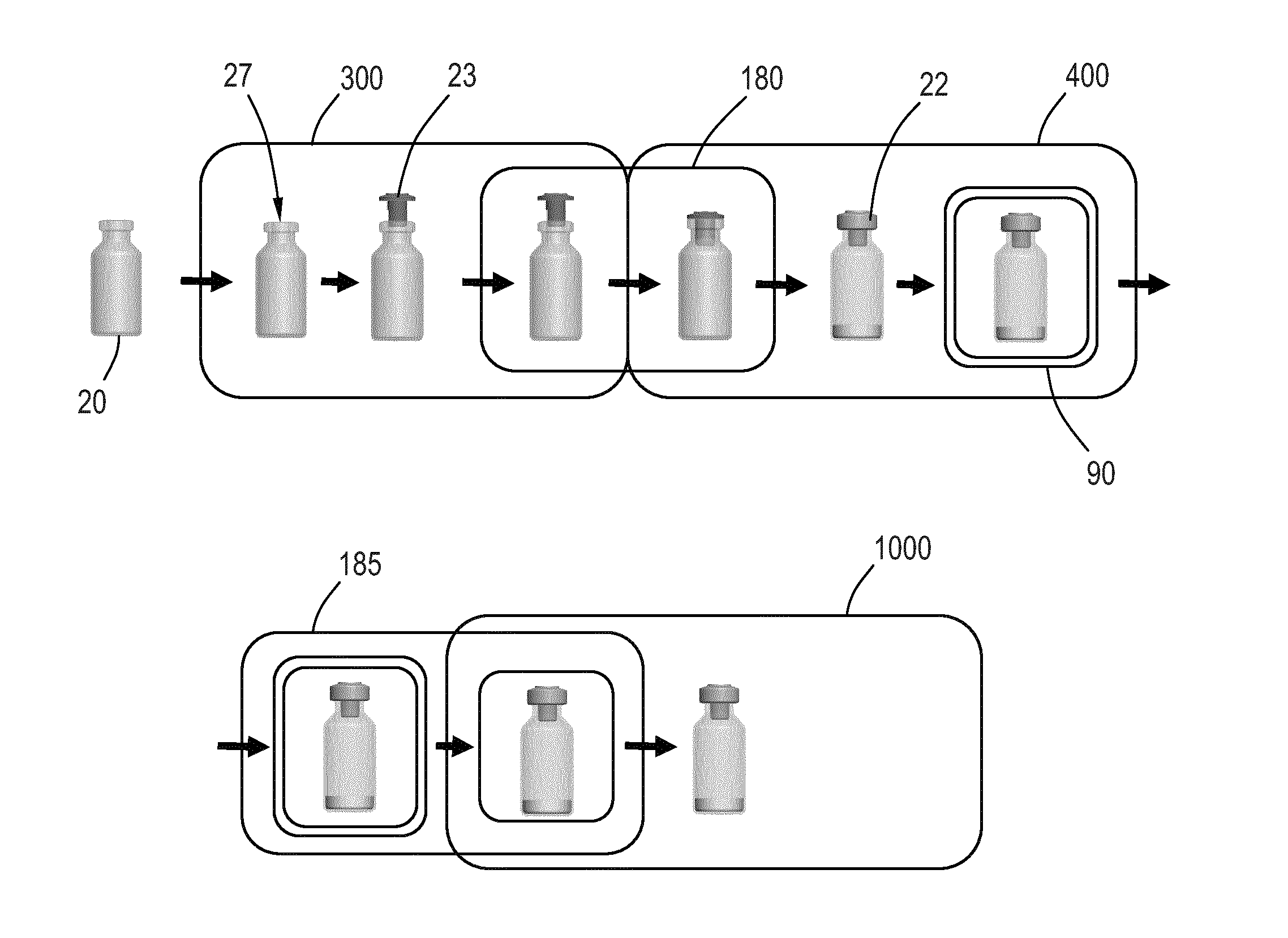
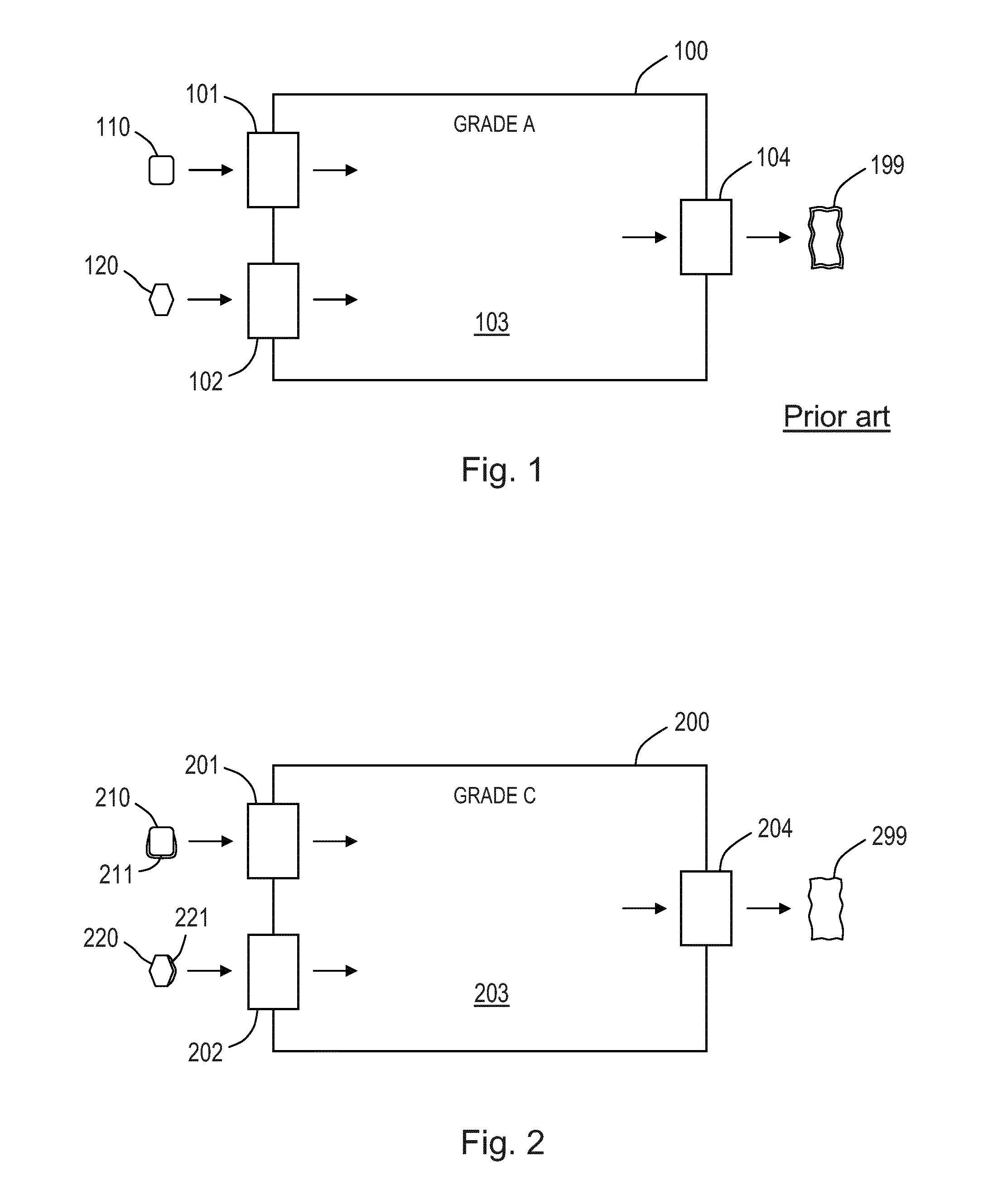
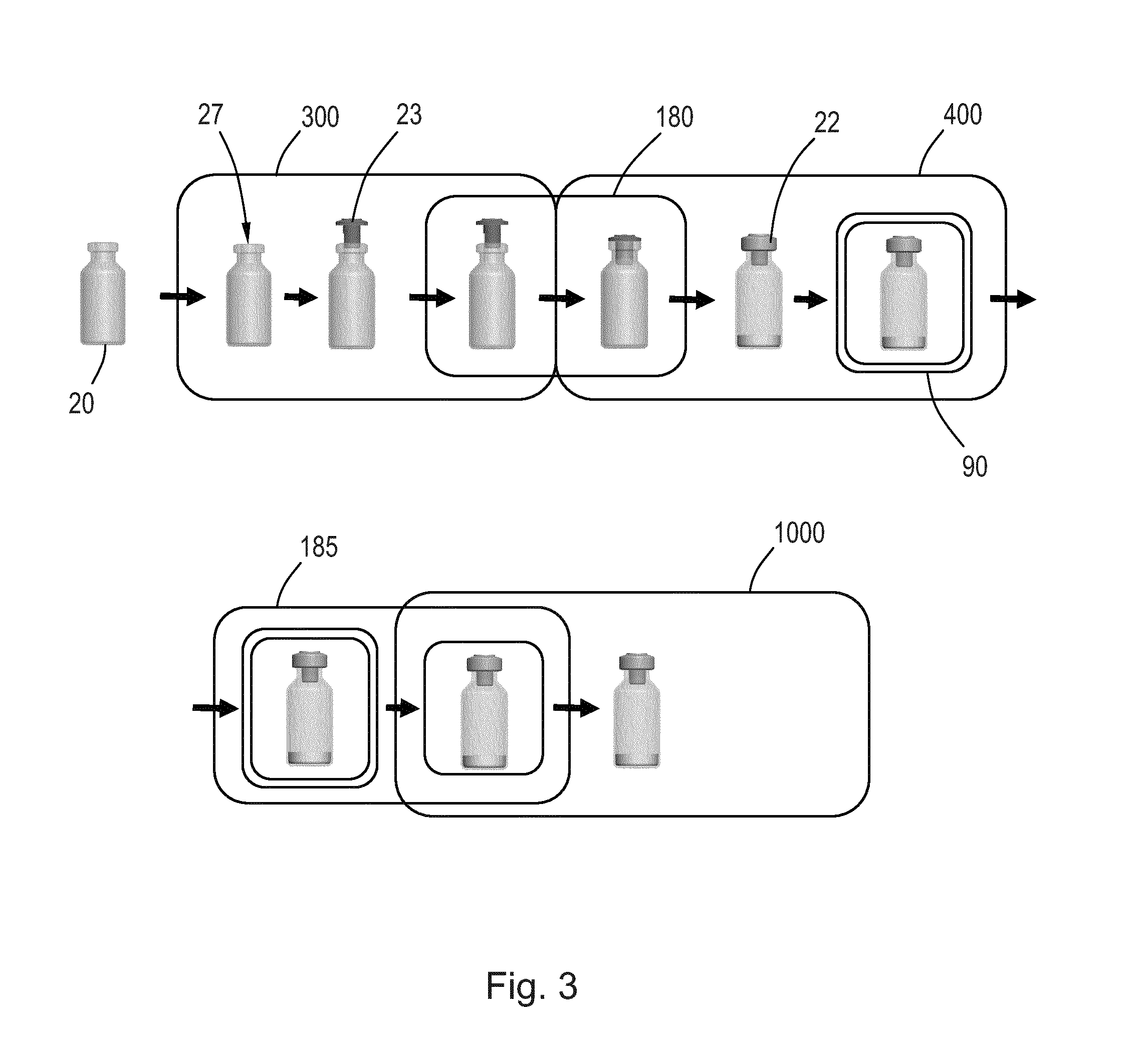
[0073]FIG. 1 shows schematically a conventional process for the manufacturing of a medical device requiring a certain level of asepsis. Different components 110, 120 for the device are pretreated, e.g. sterilised, in entry sections 101, 102 for a cleanroom environment 100 and enter a Grade A cleanroom 103 capable of maintaining an acceptably high airborne particulate cleanliness. In the cleanroom 103 the components 110, 120 are assembled and otherwise handled to produce a final device 199 which is then enclosed in a sterile packaging before leaving via an exit section 104, to thereby provide an internal particu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dimensional stability | aaaaa | aaaaa |
| Flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com