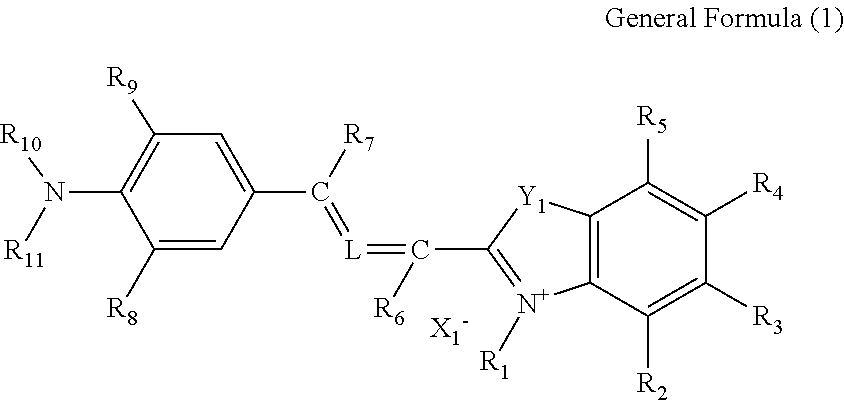
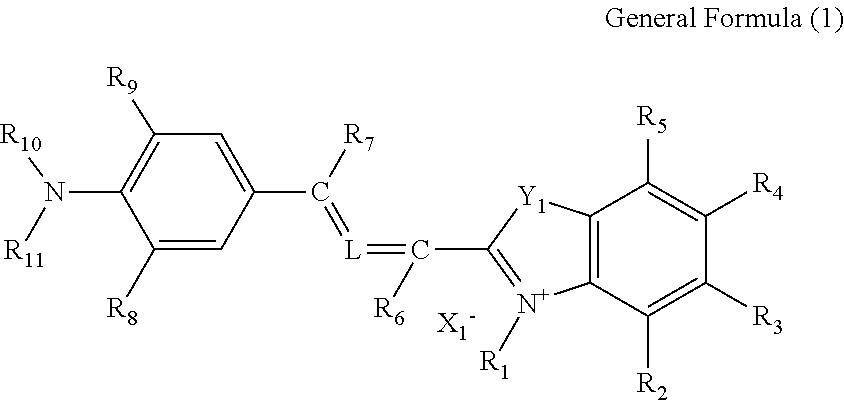
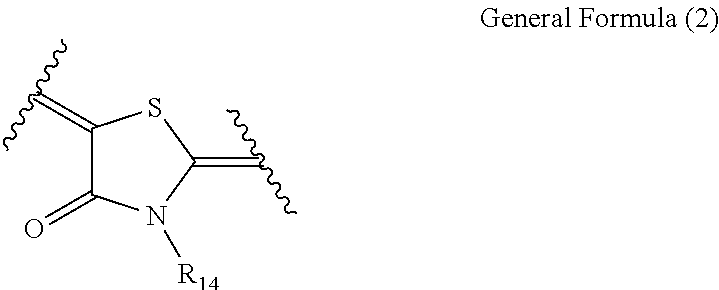
Cancer cell inhibitory drug and cancer stem-cell detection probe
a cancer stem cell and inhibitory drug technology, applied in the direction of drug compositions, organic chemistry, organic active ingredients, etc., can solve the problems of radioactive compounds affecting normal cells, safety becomes a matter of concern, etc., and achieve the effect of accurately determined and easy detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0212]Production Examples of the compounds of the present invention will be shown.
[0213]Production of Compound (1)
[0214]To a solution of compound (A) (2.4 g (11.4 mmol)) in acetic acid (20 mL), compound (B) (4.0 g (11.5 mmol)) and ammonium acetate (1.6 g) were added and stirred under reflux for 2 hours. After completion of the reaction, while the reaction solution was cooled, water (50 mL) was gently added dropwise to cool the reaction solution to room temperature. The solid substance precipitated was filtered, washed twice with water (100 mL) and further washed with 2-propanol (50 mL) to obtain the desired product (1) (3.3 g) (yield 54.4%). The desired product was confirmed by 1H nuclear magnetic resonance spectroscopic analysis (ECA-400, manufactured by JEOL Ltd.) and LC / TOF MS (LC / MSD TOF, manufactured by Agilent Technologies).
[0215]Production of Compound (15)
[0216]To a solution of compound (C) (1.4 g (5.2 mmol)) in acetic acid (20 mL), compound (D) (2.2 g (5.2 mmol)) and ammoniu...
example 2
[0221]Measurement of Fluorescent Property of Compound
[0222]A 5 μM DMSO solution of each of the compounds shown in the following Table 1 was prepared. The excitation wavelength and fluorescence wavelength of the compound were measured by a FL4500 spectrofluorometric measuring machine manufactured by Hitachi High-Technologies Corporation.
TABLE 1ExcitationFluorescenceCompoundwavelength λexwavelength λemCompound 1560619Compound 5582678Compound 8586623Compound 11614669Compound 15568643Compound 20493585Compound 26609692Compound 31611720Compound 32592671Compound 33560677Compound 34575611Compound 36488594Compound 38573670Compound 39591626Compound 40498602Compound 44588679
experimental example 1
[0223]Observation on cancer cell inhibitory (growth suppressive) action against pancreatic cancer cells Human pancreas cancer cells, KLM-1, were pre-cultured in RPMI1640 medium containing 10% FBS at 37° C. in a 5% CO2 ambient. Thereafter, 4,000 cells were seeded per well of a 96-well plate and further cultured for 24 hours. Subsequently, Compound (1) was added to the medium so as to obtain a final concentration of 10 μg / mL and cultured at 37° C. for 24 hours in a 5% CO2 ambient. The cultured cells were analyzed for viable cell count according to CellTiter-Glo Luminescent Cell Viability Assay (manufactured by Promega KK.). As a reference, the number of cells cultured in a medium containing a 0.1% dimethylsulfoxide solution (hereinafter, simply referred to as DMSO) in place of a medium containing Compound (1), in the aforementioned operation, was regarded (100%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inhibition | aaaaa | aaaaa |
| Luminescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com