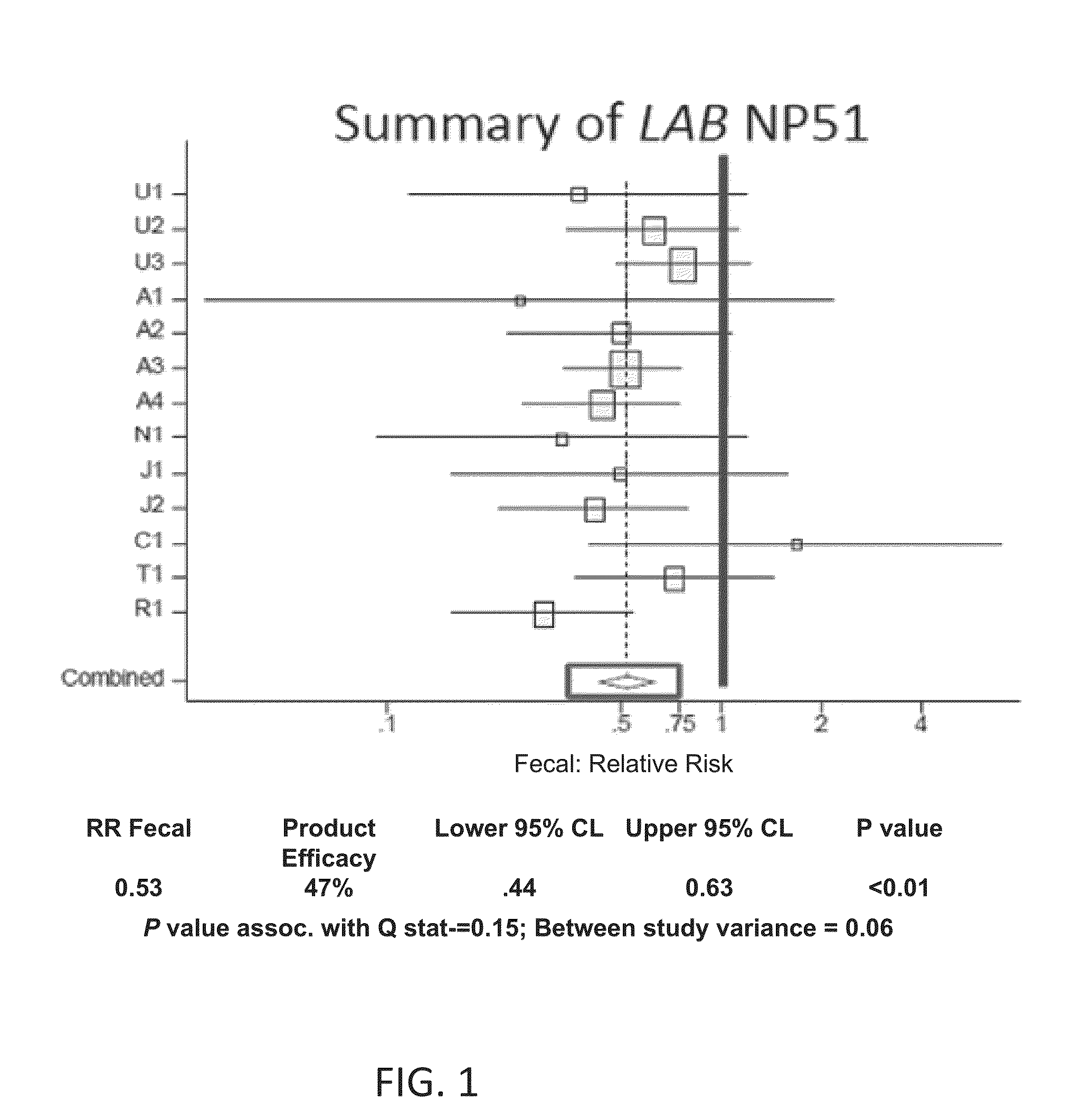
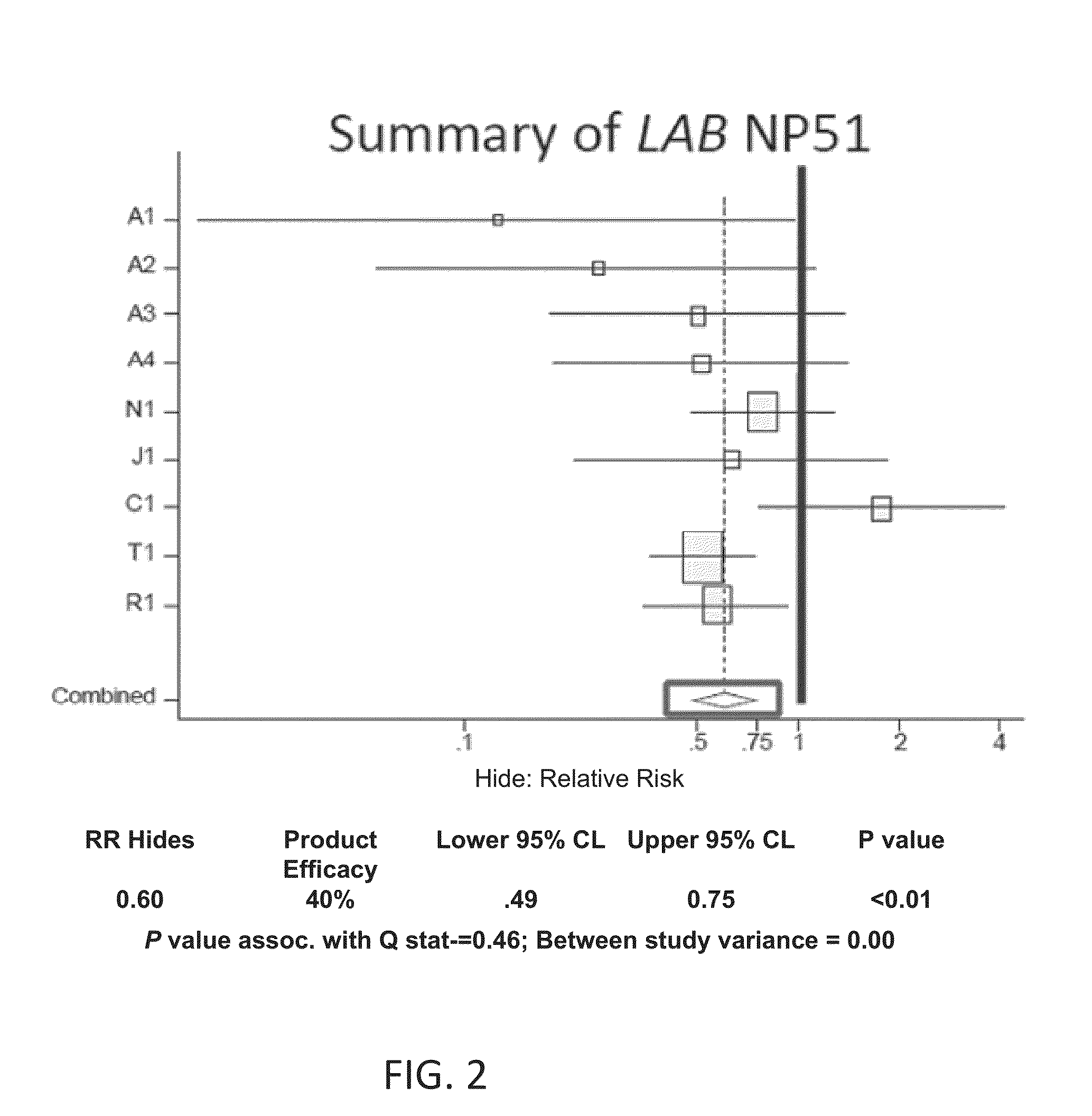
Low/High Dose Probiotic Supplements And Methods Of Their Use
a probiotic and high-dose technology, applied in the field of lactic acid bacteria, can solve problems such as poor feed performance, and achieve the effects of reducing pathogenic infection, enhancing feed performance, and reducing pathogenic infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Low Dose / High Dose Feeding of Lactobacillus acidophilus / animalis in Combination with a Fixed Dosage of Propionibacterium freudenreichii
[0070]Beef cattle are fed with normal feed such as steam-flaked corn-based diet. Sixty days prior to when the cattle are scheduled to be harvested, all animals start to receive a low dose supplemental DFM in addition to their normal feed. The low dose DFM contains Lactobacillus acidophilus / animalis strain LA51 and Propionibacterium freudenreichii strain PF24 in an amount such that each animal's intake of the Lactobacillus acidophilus / animalis strain LA51 is about 1×107 CFU per day and the intake of Propionibacterium freudenreichii strain PF24 is about 1×109 CFU per day.
[0071]After 30 days on the low dose DFM supplement, the cattle are switched to a feed containing a high dose supplemental DFM in addition to the normal feed. During this high dose period which lasts about 30 days before the animals are slaughtered, the daily intake of Propionibacteriu...
example 2
Low Dose / High Dose Feeding of Lactobacillus acidophilus / animalis Alone to Maximize Feed Performance and Pathogen Reduction
[0072]Beef cattle are fed with normal feed such as steam-flaked corn-based diet. About one hundred and eighty days prior to when the cattle are scheduled to be harvested, all animals start to receive a low dose supplemental DFM in addition to their normal feed. The low dose DFM contains Lactobacillus acidophilus / animalis strain LA51 in an amount such that each animal's intake of the Lactobacillus acidophilus / animalis strain LA51 is about 1×107 CFU per day.
[0073]After 150 days on the low dose DFM supplement, the cattle are switched to a feed containing a high dose supplemental DFM in addition to the normal feed. During this high dose period which lasts about 30 days before the animals are to be slaughtered, the daily intake of Lactobacillus acidophilus / animalis strain LA51 is increased to about 1×109 CFU per day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com