Innate immune proteins as biomarkers for CNS injury
a technology of innate immune proteins and biomarkers, applied in the field of neurology, immunology, diagnostics, etc., can solve the problems of one-third of injury-related deaths, difficult to predict the severity and outcome of tbi and sci, and limited valu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Inflammasome Proteins are Secreted into Cerebrospinal Fluid after Spinal Cord Injury
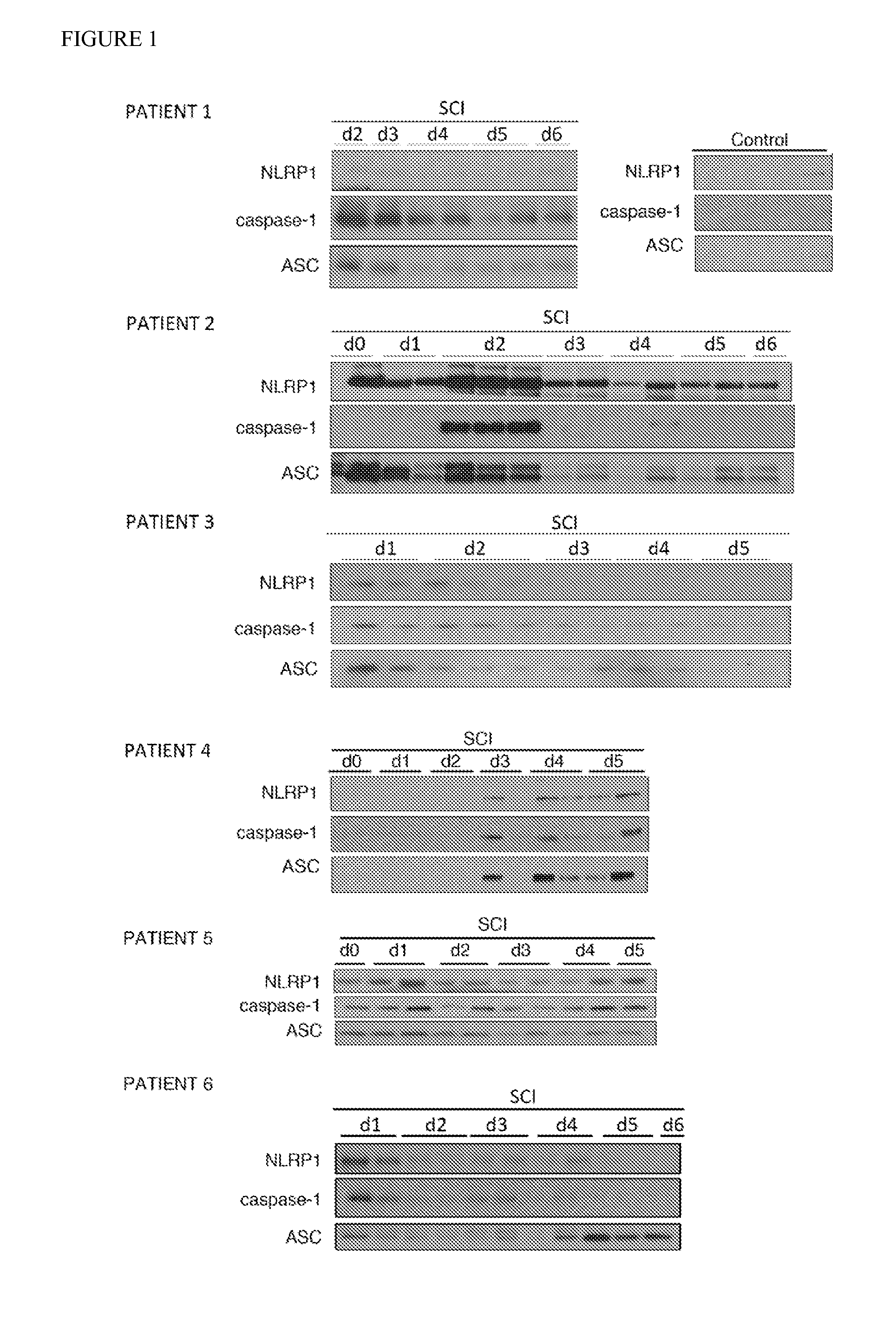
[0043]To determine whether NLRP1 inflammasome proteins were present in cerebrospinal fluid (CSF) following spinal cord injury (SCI), CSF samples from seven patients with SCI or control patients were analyzed for levels of nucleotide-binding leucine-rich repeat pyrin domain containing protein 1 (NLRP1; also known as NAcht leucine-rich-repeat protein 1 (NALP-1)), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and caspase-1. The American Spinal Cord Injury Association (ASIA) scale of the SCI patients at admission to the emergency department ranged from AIS A to B. Information regarding the diagnosis, procedures and outcomes of the patients is shown in Table 1. None of the patients had any complications. CSF from uninjured individuals was obtained as a control from three males and two females ranging from 67 to 91 years old.
[0044]For detection of inflammasome prote...
example 2
Immunohistochemical Expression of NLRP1 Inflammasome Proteins in Spinal Cords after Injury
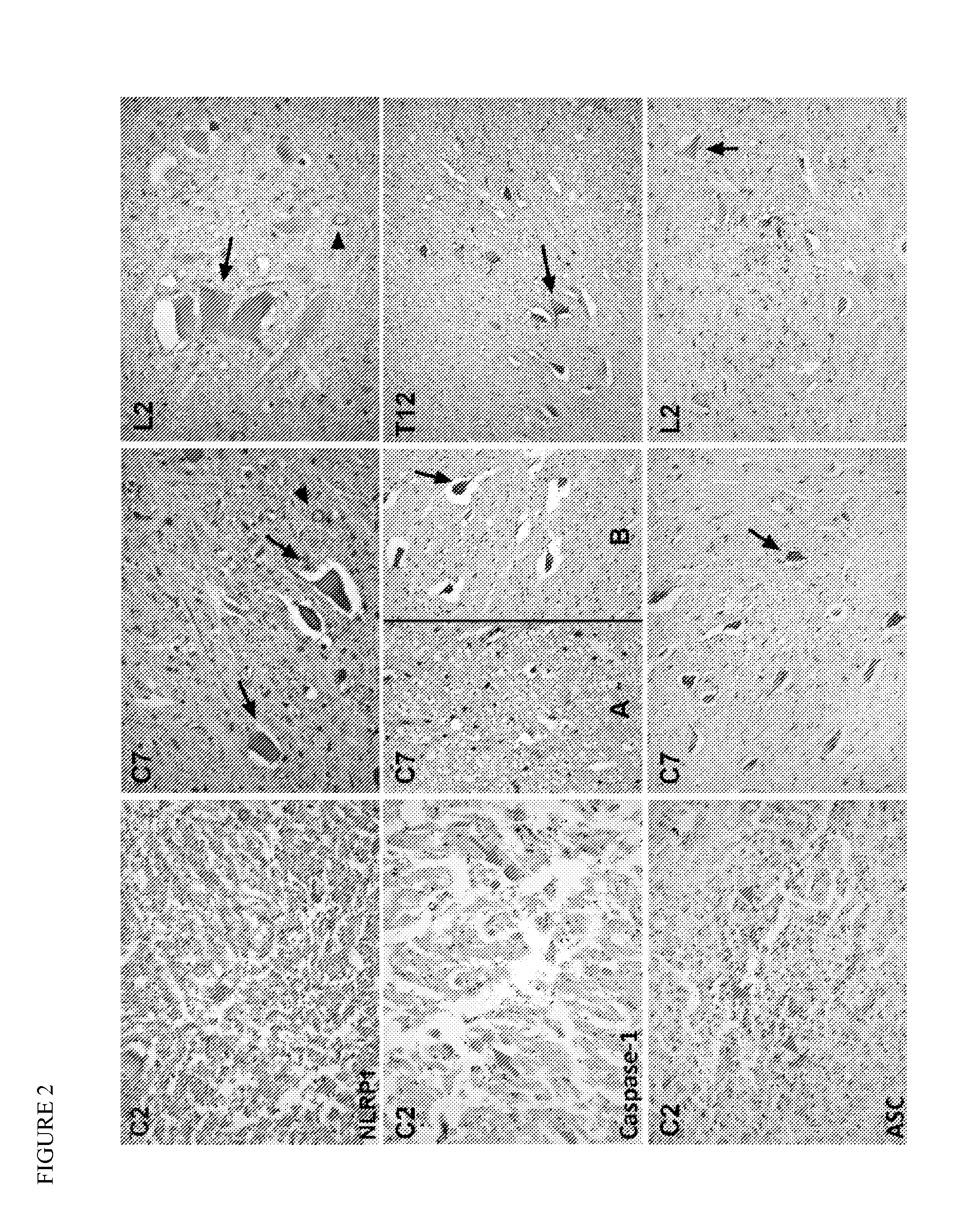
[0047]Spinal cord sections were obtained from nine decedents (8 males and 1 female with ages ranging from 20 to 77 years) who had injury to the spinal cord due to vertebral fractures. The spinal cord injury was assessed microscopically, using bright field optics, by examining one H&E or H&E / DAB-stained section from the lesion center of each case or from cervical, thoracic and lumbar sections from control cases. The spinal cord injuries were classified on the basis of their histological appearance as “contusion / cyst,” massive compression, or laceration (Fleming et al., 2006). Contusional injuries were characterized by an intact pia and relative preservation of the anatomical relations of various elements of the spinal cord, and variable degrees of injury ranging from involvement of the entire cross-sectional area to large usually asymmetric areas of tissue damage. Massive compression injuries we...
example 3
Inflammasome Proteins in Cerebrospinal Fluid of Brain-Injured Patients are Biomarkers of Functional Outcome
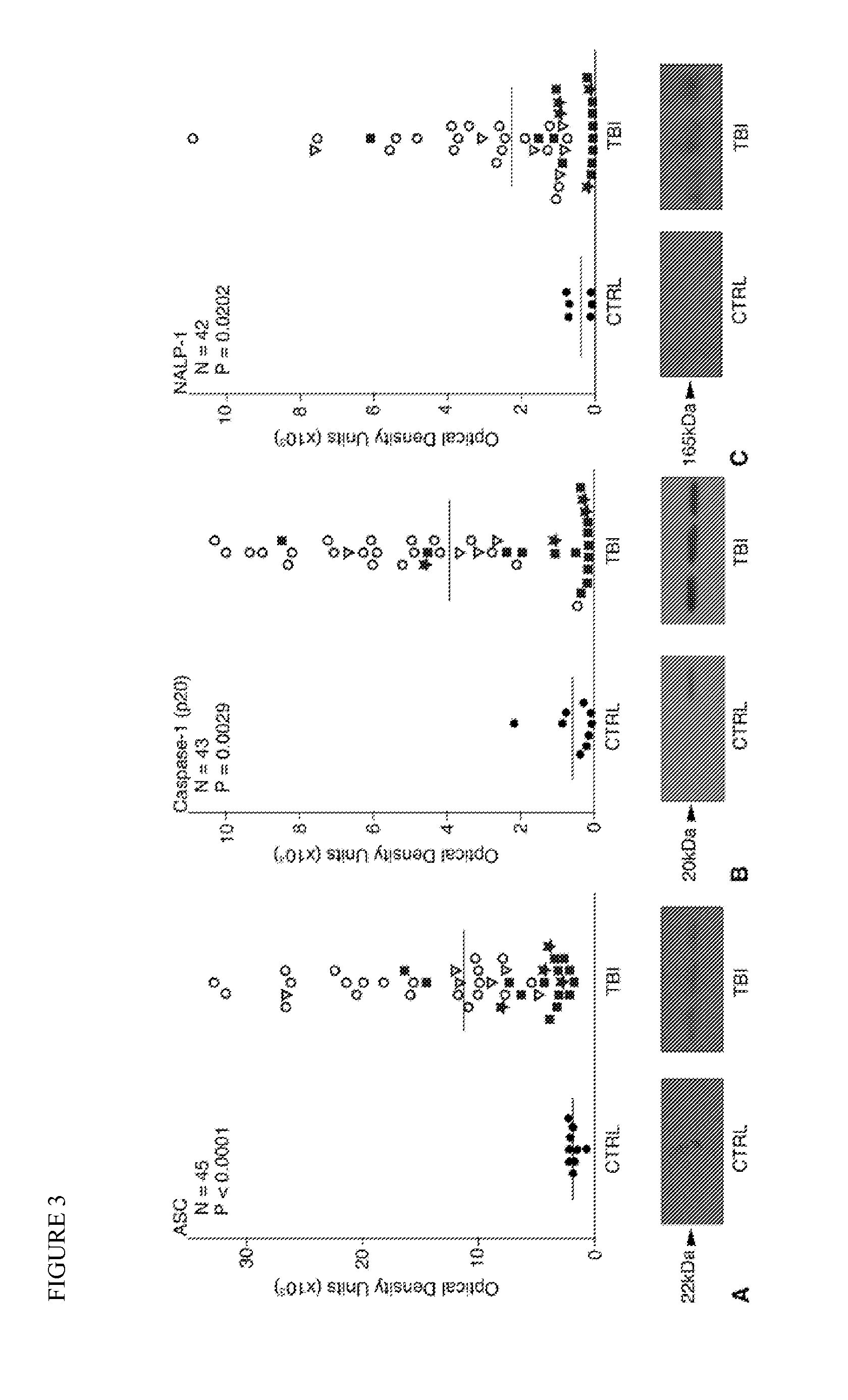
[0054]To determine whether inflammasome proteins may serve as biomarkers for other types of central nervous system injury, a total of 45 CSF samples were collected from 23 traumatic brain injury (TBI) patients on the day of injury and up to three days after the injury and analyzed by immunoblot for levels of NALP-1 (also known as NLRP1), ASC, and caspase-1. Each of the patients presented with the following inclusion criteria: severe or moderate head trauma (Glasgow Coma Scale (GCS) score≦12), age 1 month to 65 years, and ventriculostomy. Twenty-two of the patients suffered severe brain trauma (GCS score≦8) and 1 suffered moderate brain trauma (moderate TBI GCS score range 9-12). Nine patients (5 men and 4 women) with a mean age of 66.3 years (range 29-91 years) served as controls. Control patients required a ventriculostomy for nontraumatic pathology. Patients with acute mening...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thick | aaaaa | aaaaa |
| acidic | aaaaa | aaaaa |
| light microscopy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com