Stable Formulations of Linaclotide
a technology of linaclotide and stable formulation, which is applied in the field of stable pharmaceutical compositions of linaclotide, can solve problems such as exacerbated difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
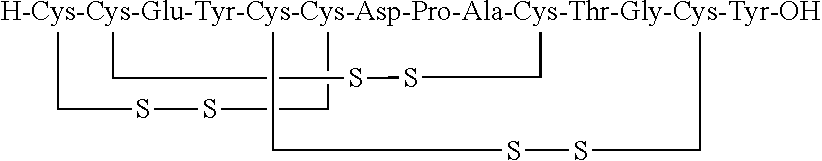
[0119]Linaclotide beads were prepared in the following manner using the components set forth in Table 1. First, a linaclotide solution was prepared by combining linaclotide, polyvinyl alcohol, calcium chloride, meglumine and water in the concentrations set forth in Table 1. The linaclotide solution was then pH-adjusted to about 2.5 and mixed until clear. Next, the linaclotide solution was layered onto isomalt beads by spraying the beads with the linaclotide solution using a Wurster process. The linaclotide-layered beads were then dried until the product loss on drying (LOD) was less than about 3%.
TABLE 1Linaclotide beads, 5 μg / 50 mgWeightComponents(g)Wt. %Linaclotide0.240.012Isomalt191595.8Meglumine2.60.1Calcium chloride dihydrate2.00.1PVA804HClQ.S.QSPurified water*1000QSTOTAL2000100.0*Water is removed during the manufacturing process
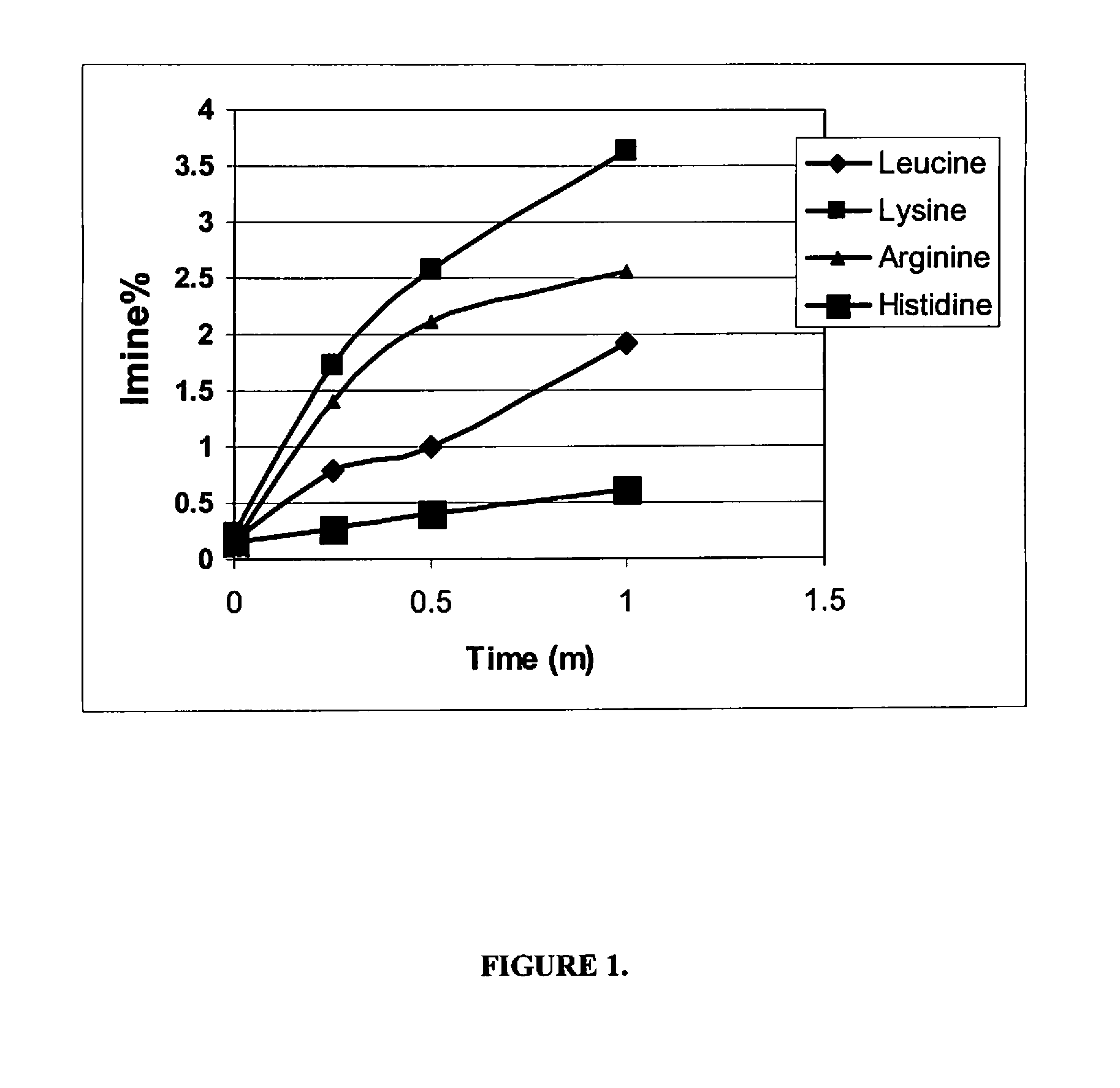
[0120]The stability performance of the linaclotide beads were assessed following storage of the beads for 1 month at 40° C. and 75% RH in 45 cc HDPE bo...
example 2
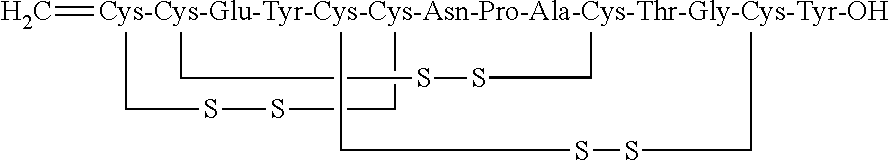
[0121]Linaclotide beads were prepared in the following manner using the components set forth in Table 3. First, a linaclotide solution was prepared by combining linaclotide, polyvinyl alcohol, calcium chloride, histidine and water in the concentrations set forth in Table 3. The linaclotide solution was then pH-adjusted to about 2.5 and mixed until clear. Next, the linaclotide solution was layered onto isomalt beads by spraying the beads with the linaclotide solution using a Wurster process. The linaclotide-layered beads were then dried until the product loss on drying (LOD) was less than about 3%.
TABLE 3Linaclotide beads, 5 μg / 50 mgWeightComponents(g)Wt. %Linaclotide0.240.012Isomalt191695.8Histidine20.1Calcium chloride dihydrate2.00.1PVA804HClQ.S.—Purified water*1000—TOTAL2000100.0*Water is removed during the manufacturing process
example 3
[0122]Linaclotide beads were prepared in the manner described in Example 2 using the components set forth in Table 4.
TABLE 4Linaclotide beads, 5 μg / 50 mgWeightComponents(g)Wt. %Linaclotide0.240.012Isomalt191695.8Leucine20.1Calcium chloride dihydrate2.00.1PVA804HClQ.S.—Purified water*1000—TOTAL2000100.0*Water is removed during the manufacturing process
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt. % | aaaaa | aaaaa |
| wt. % | aaaaa | aaaaa |
| wt. % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com