Compounds for proteasome enzyme inhibition
a technology of proteasome and enzyme, applied in the field of compound and method of enzyme inhibition, can solve the problems of generally lacking the specificity, stability, or potency necessary to explore and exploit the role of the proteasome at the cellular and molecular level, and achieve the effects of inhibiting or reducing hiv infection in a subject, reducing the rate of muscle protein degradation, and affecting the level of viral gene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
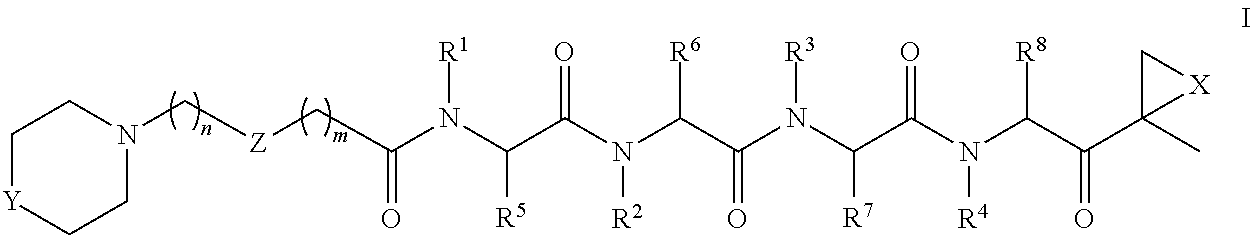
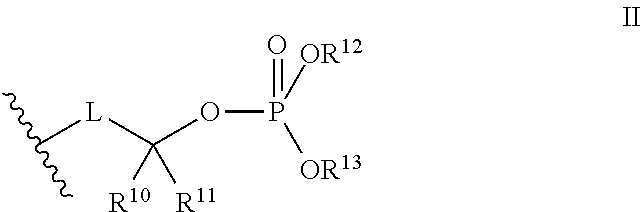
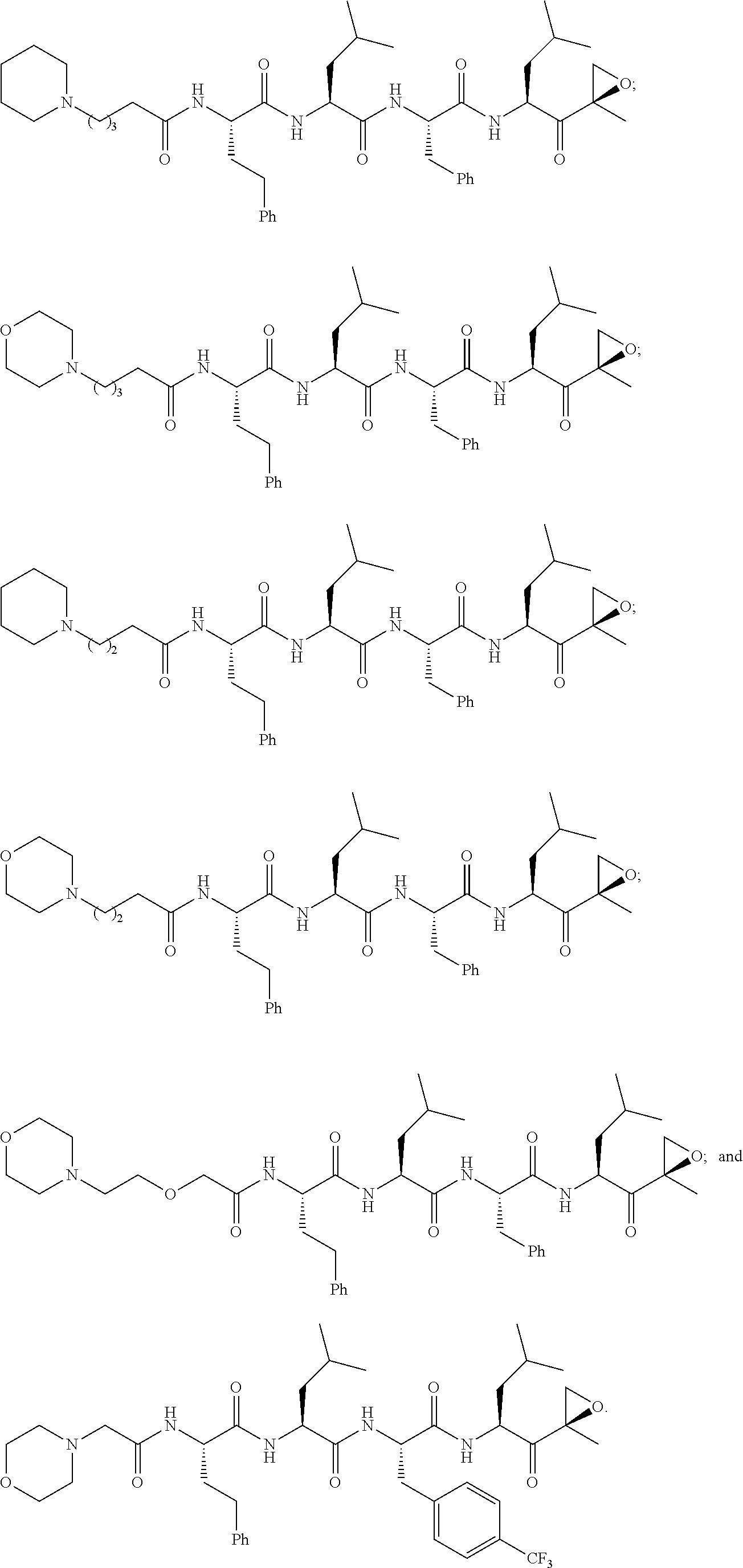
[0014]The invention involves compounds useful as enzyme inhibitors. These compounds are generally useful to inhibit enzymes having a nucleophilic group at the N-terminus. For example, activities of enzymes or enzyme subunits having N-terminal amino acids with nucleophiles in their side chains, such as threonine, serine, or cysteine can be successfully inhibited by the enzyme inhibitors described herein. Activities of enzymes or enzyme subunits having non-amino acid nucleophilic groups at their N-termini, such as, for example, protecting groups or carbohydrates, can also be successfully inhibited by the enzyme inhibitors described herein.
[0015]While not bound by any particular theory of operation, it is believed that such N-terminal nucleophiles of Ntn form covalent adducts with the epoxide functional group of the enzyme inhibitors described herein. For example, in the β5 / Pre2 subunit of 20S proteasome, the N-terminal threonine is believed to irreversibly form a morpholino or piperaz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com