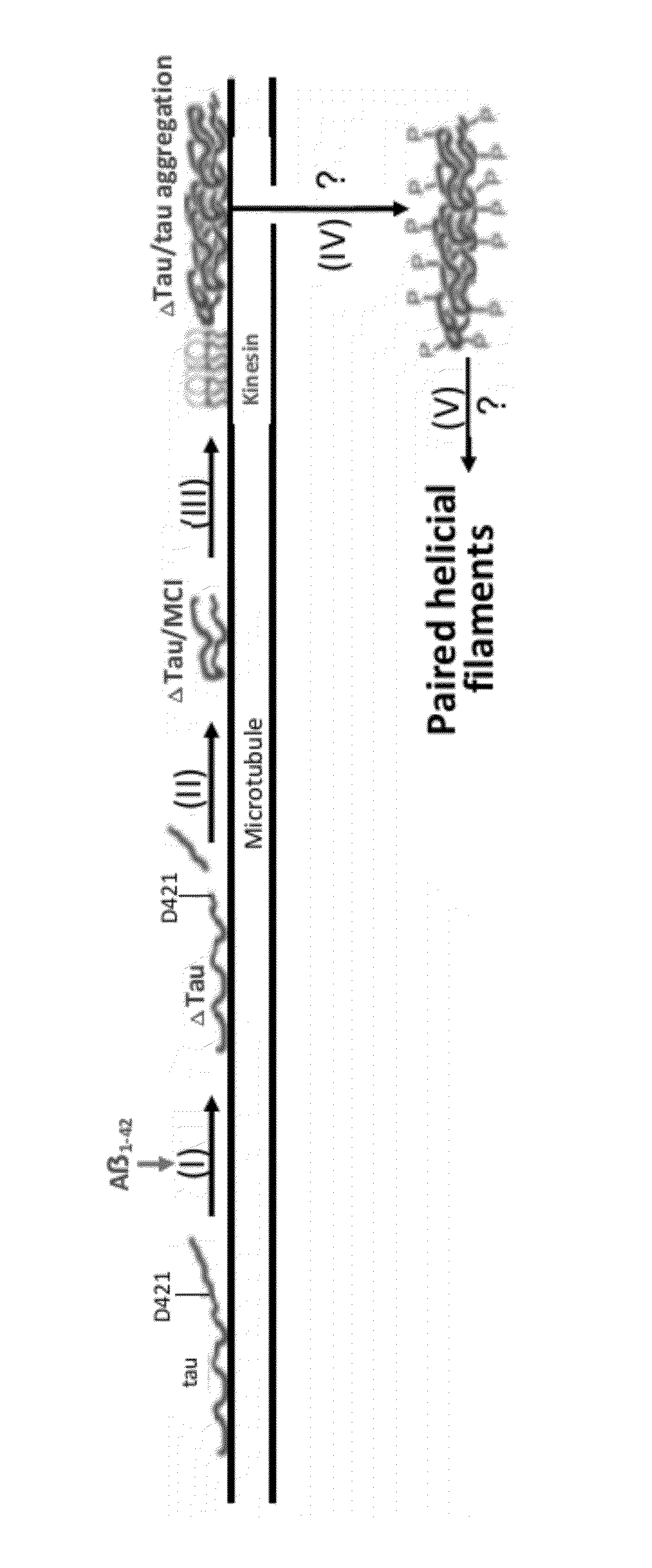
Treatment of tauopathies
a tauopathies and tau protein technology, applied in the field of tauopathies, can solve the problems of high suspicion of aggregation of tau protein in ad, achieve no binding and/or reactivity, minimize or prevent the formation of neurofiblary tangles, and slow the progression of a tangle-related behavioral phenotyp
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0279]The strategy and the protocols for generating antibodies which specifically recognize the neoepitope created by cleavage of tau at Asp421 (i.e., ΔTau), but not full length tau (i.e., htau40) is described in this prophetic example.
[0280]The following peptides are prepared using an Applied Biosystems Peptide Synthesizer (430A): a peptide corresponding to tau416-421 (SEQ ID NO:83 SIDMVD); a peptide corresponding to tau417-421 (SEQ ID NO: 84 IDMVD); a peptide corresponding to tau 418-421 (SEQ ID NO:85 DMVD); and a peptide corresponding to SEQ ID NO:86, all of htau40.
[0281]The synthetic peptides are then purified by HPLC and characterized using amino acid composition.
[0282]Once purified and characterized, the peptides are conjugated to keyhole limpet hemocyanin, and four sets 10 Balb / c mice are immunized with the conjugated peptides.
[0283]Following the completion of the immunization, a fusion procedure is performed using spleenoxytes from the hyperimmunized mice and an appropriate ...
example 2
[0289]In Example 2, the therapeutic potential of a vaccine against Aβ, tau, and in combination in a triple transgenic mouse model of Alzheimer's disease (3×Tg-AD) that expresses both plaque and tangle pathology is evaluated. A first goal of Example 2 is to evaluate such vaccines as a preventative against AD neuropathology and cognitive decline. The second goal of Example 2 is to evaluate such vaccines as a therapeutic once AD neuropathology is already established. In Example 2, the assignee's RECALL-Vax vaccine technology is utilized.
[0290]The triple transgenic mouse model contains 3 mutations relevant to Alzheimer's pathology (PS1M146V, βAPPSwe, and tauP301L) (Oddo et al., 2003). (Dr. Frank LaFerla, UC Irvine). The mice were generated by microinjecting two transgenes (βAPPSwe, and tauP301L) into a single cell embryo from a homozygous presenilin-1 knock-in animal. The presenilin-1 knock-in gene contains the mutation M146V which increases the amount of Aβ1-42 produced relative to Aβ1...
example 3
[0315]In Example 3, the safety and efficacy of an anti-Δ tau vaccine, an anti-Aβ vaccine and a combination anti-Δ and anti-Aβ vaccine will be accessed. The anti-Δ tau vaccine will comprise an immunogenic peptide of SEQ ID NO. 116 (H2N-VDDALINSTKIYSYFPSVGPSLIDMVD-OH) and alum. The anti-Aβ vaccine will comprise an immunogenic peptide of SEQ ID NO. 117 (H2N-DAEFGPSLVDDALINSTKIYSYFPSV-OH) and allum. The combination vaccine will comprise a mixture of the immunogenic peptides of SEQ ID NOS. 116 and 117, and alum. In these vaccines, alum will be used as adjuvant, and immunogenic peptides will be used active agents.
[0316]Each vaccine will be administered to a group of LaFerla mice (i.e., transgenic mice expressing 3 mutations relevant to Alzheimer's pathology (PS1M146V, βAPPSwe, and tauP301L)). There would also be a control group of mice which will not receive any vaccines containing peptides of SEQ ID NO: 116 or SEQ ID NO 117. The “vaccine” administered to the control group will comprise a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| equilibrium constant | aaaaa | aaaaa |
| equilibrium constant | aaaaa | aaaaa |
| equilibrium constant KD | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com