Polypeptide structural motifs associated with cell signaling activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Structural Motif within a GlyRS Polypeptide Fragment Having Cell Signaling Activity
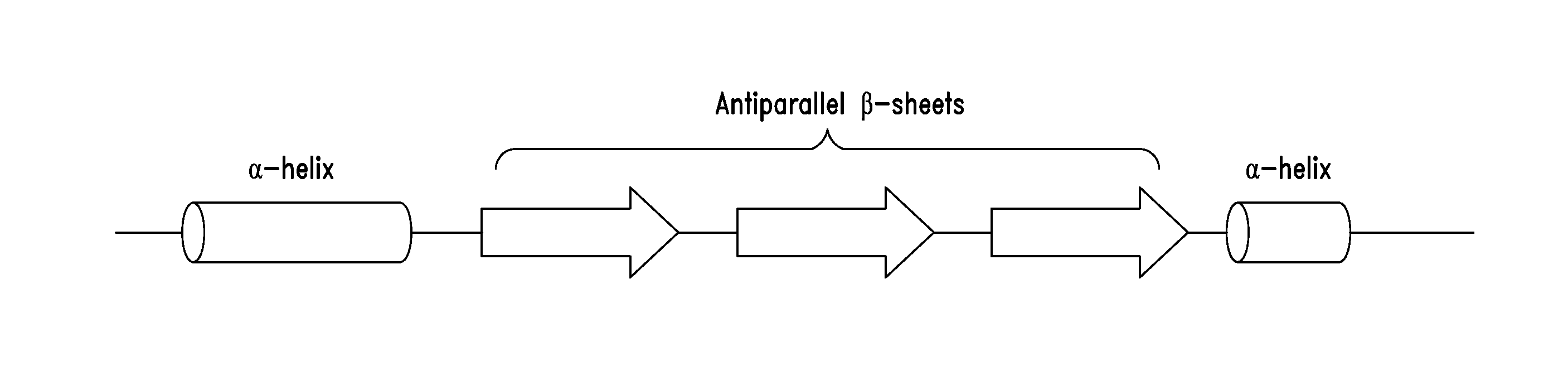
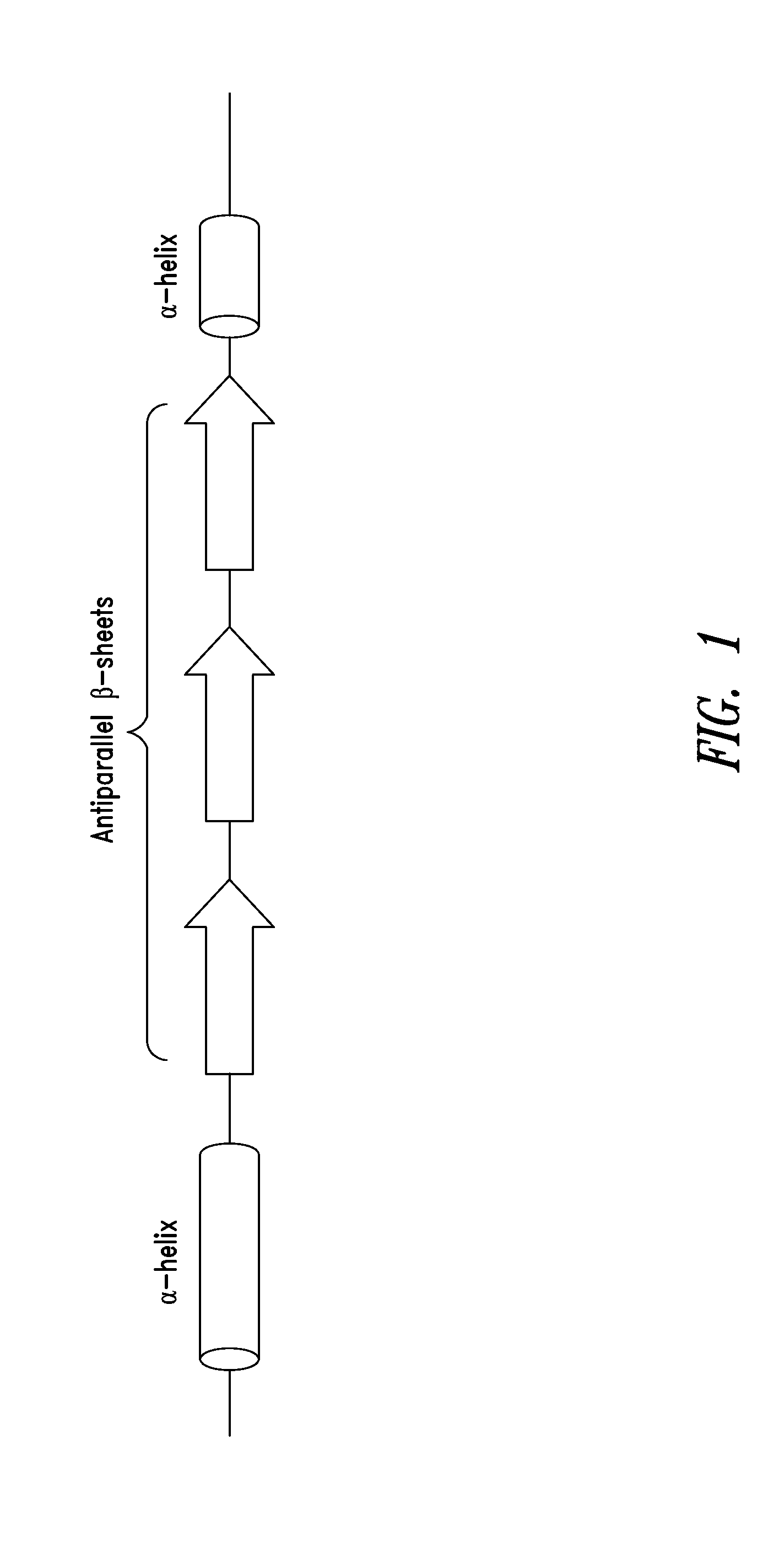
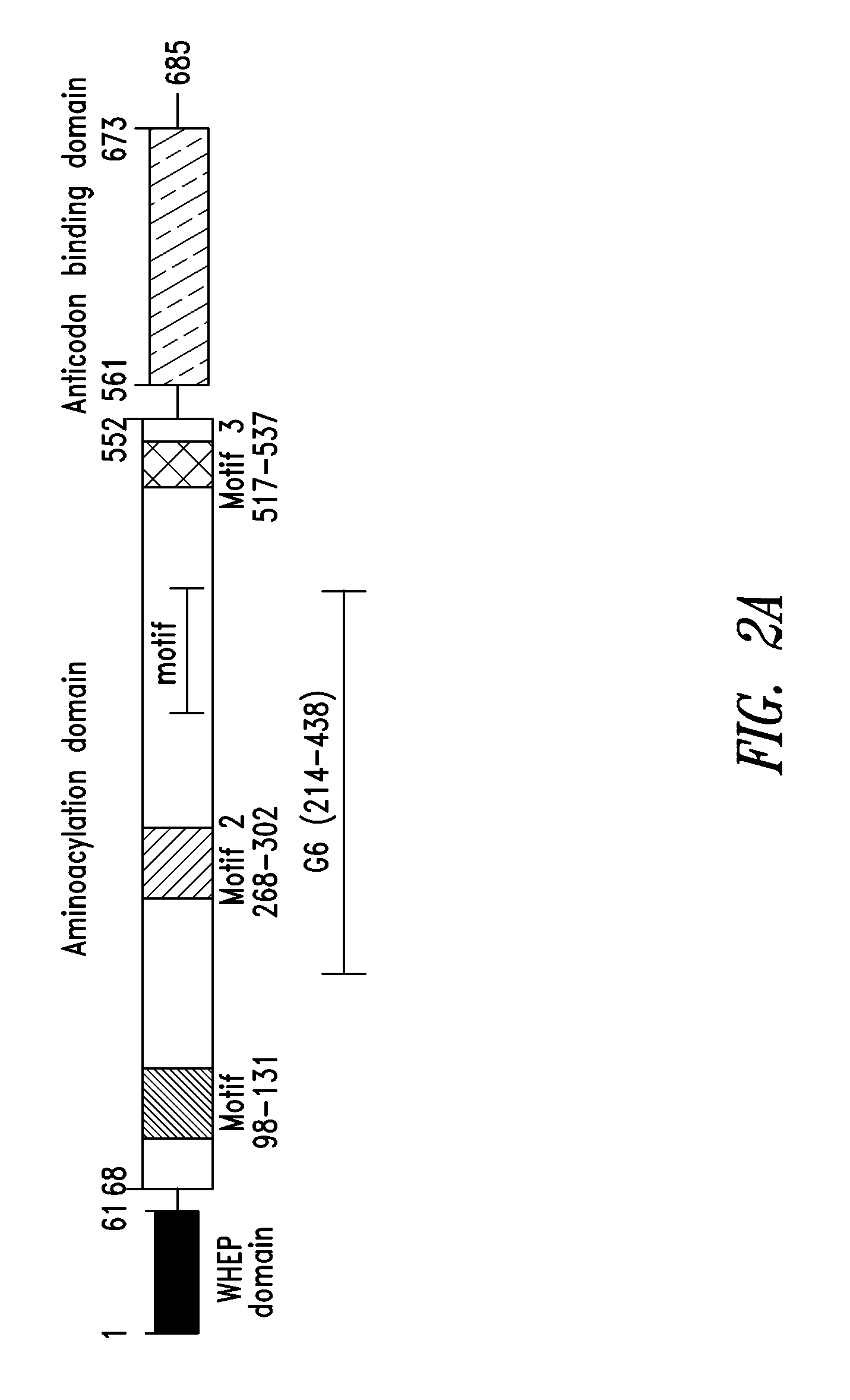
[0297]As shown in FIGS. 2A-2C, a fragment of human glycyl-tRNA synthetase (GlyRS) referred to as G6, and corresponding to residues 214-439 of human GlyRS (SEQ ID NO: 1), was unexpectedly discovered to be effective in inducing GPCR-dependent chemotaxis of THP-1 monocytes. Further analysis of this active fragment revealed the presence of a structural motif, comprising 3 antiparallel β-sheets flanked by an α-helix at each end, believed to be responsible for the observed chemokine activity. The structural motif was identified as being contained within amino acid residues 345-438 of the human GlyRS protein sequence set forth in SEQ ID NO: 1.
example 2
Identification of Structural Motif in Other Class II AARS Proteins
[0298]The structural motif identified above in Example 1 was also found in similar regions of other human Class II AARS. These included AspRS, HisRS, ThrRS, GluProRS and SerRS. This region of Class II AARS proteins is highly conserved structurally among members of this AARS class indicating that the region contains the same basic structural architecture, but is not well conserved in residue sequence identity, creating the potential to display different sequences which may have different signaling activities on this architecture. (e.g., FIG. 4, adapted from O'Donoghue and Luthey-Schulten, Microbiology and Molecular Biology Reviews, December 2003)
[0299]The structural motif was identified as being contained within amino acid residues 367-448 of the human AspRS protein sequence set forth in SEQ ID NO: 2; within amino acid residues 294-372 of the human HisRS protein set forth in SEQ ID NO: 3; within amino acid residues 469...
example 3
Illustrative Non-AARS Proteins Containing the Structural Motif
[0300]Further analysis identified the presence of this same structural motif comprising 3 antiparallel β-sheets and two α-helices in several non-AARS proteins (FIG. 5). For example, the motif was identified in human thioredoxin, contained within residues 20-105 of the human thioredoxin sequence of SEQ ID NO: 11, as well as within residues 20-84 of Trx80, which is a truncated, secreted form of thioredoxin containing the N-terminal 84 amino acid residues.
[0301]The motif was also identified in the human macrophage inhibitory factor protein, contained within residues 1-90 of the human thioredoxin sequence of SEQ ID NO: 12.
[0302]The motif was also identified in the human peroxiredoxin 5 isoform B protein, contained within non-contiguous fragments corresponding to residues 32-68 and 125-161 of the human peroxiredoxin 5 isoform B sequence of SEQ ID NO: 13.
[0303]Thus, by screening non-AARS proteins for the presence of the identif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com