Polymer metal and composite implantable medical devices
a medical device and metal composite technology, applied in the field of polymer metal and composite implantable medical devices, can solve the problems of large profile, difficult visualization of stents during delivery and after deployment, and inability to bioerode, and achieve the effect of different erosion ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
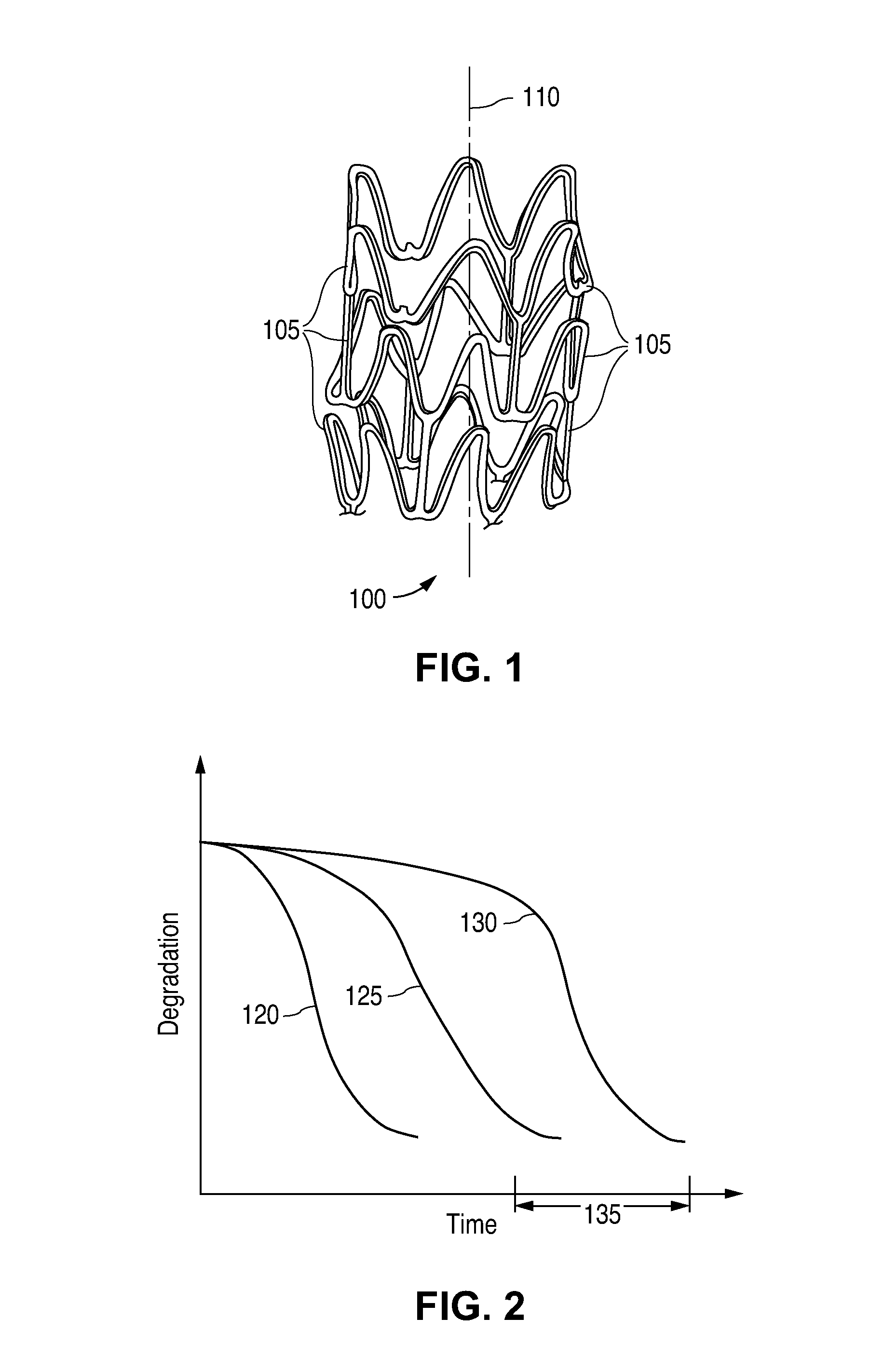
[0021]The term “implantable medical device” is intended to include self-expandable stents, balloon-expandable stents, stent-grafts, and grafts. The structural pattern of the device can be of virtually any design. A stent, for example, may include a pattern or network of interconnecting structural elements or struts. FIG. 1 depicts a three-dimensional view of a stent 100 which shows struts 105. The implantable medical device has a cylindrical axis 110. The pattern shown in FIG. 1 should not be limited to what has been illustrated as other stent patterns are easily applicable with the method of the invention. A stent such as stent 100 may be fabricated from a tube by forming a pattern with a technique such as laser cutting or chemical etching.
[0022]Various embodiments of the present invention relate to implantable medical devices and methods of manufacturing such devices that possess desired combinations and degrees of properties such as radial strength, flexibility, radio-opacity, lo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| erosion | aaaaa | aaaaa |
| surface eroding | aaaaa | aaaaa |
| erosion rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com