Mammalian receptors as targets for antibody and active vaccination therapy against mold infections
a technology of mammalian receptors and active vaccination therapy, which is applied in the field of compositions and methods for treating and preventing infectious diseases in patients, can solve the problems mucormycosis, and human fungal infection, and the effect of nephrotoxicity and other adverse effects, and the use of antifungal therapy alone is rarely curativ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
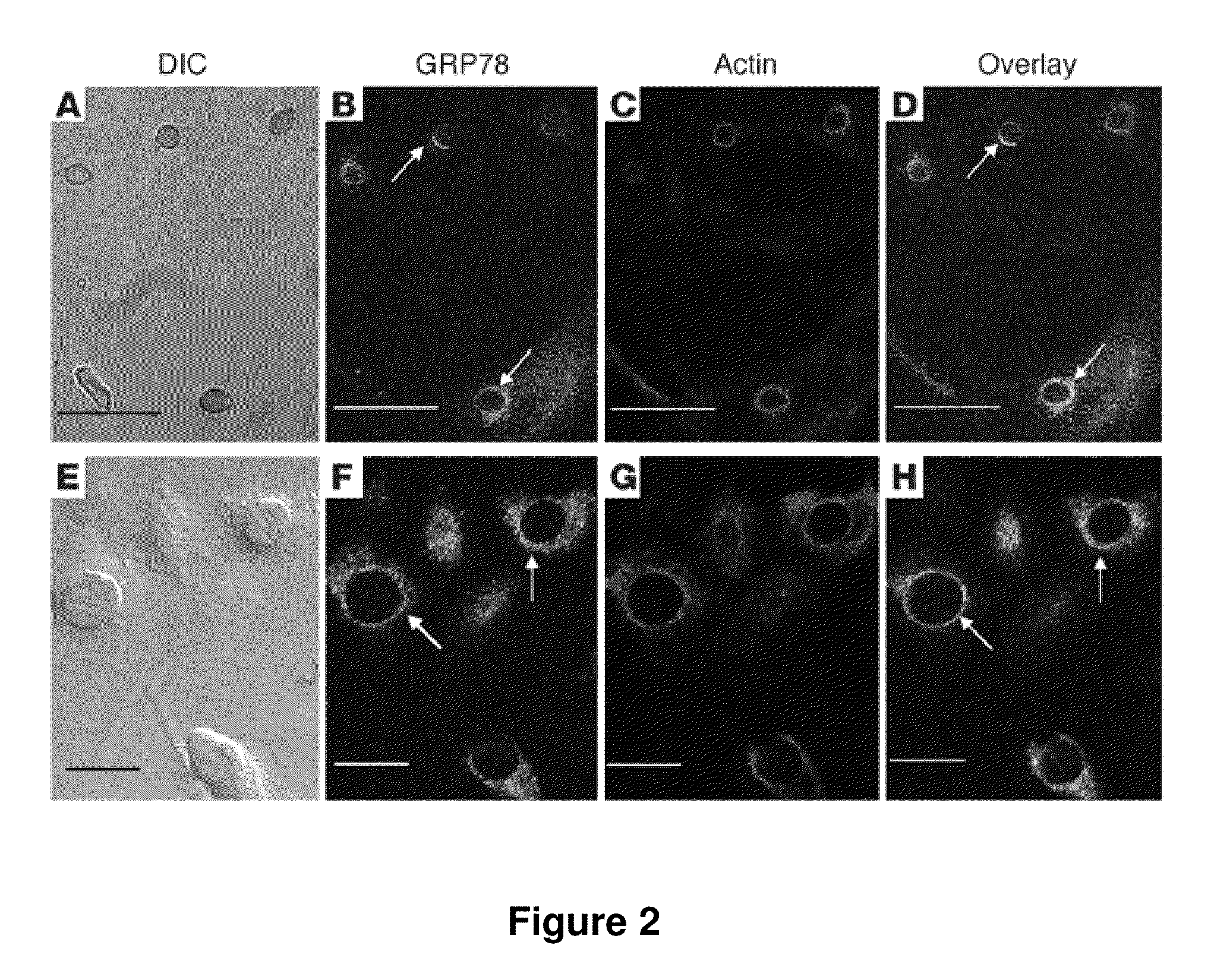
Endothelial Cell Receptor GRP78 is Required for Mucormycosis Pathogenesis
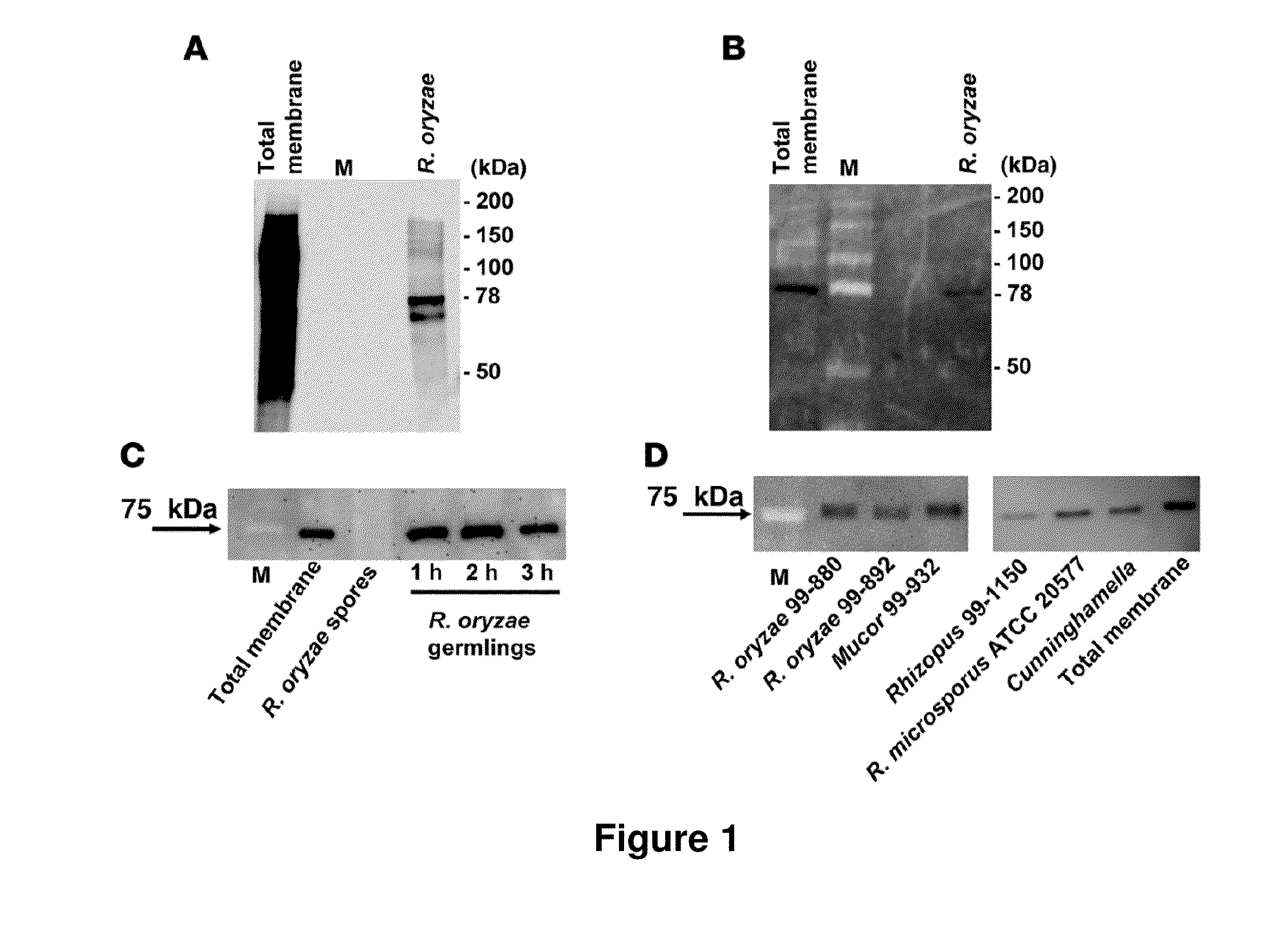
[0122]Mucormycosis is a life-threatening infection caused by fungi of the order Mucorales, the most common etiologic species of which is Rhizopus oryzae. The most common predisposing risk factor for mucormycosis is diabetes mellitus, and it has been long established that patients with diabetic ketoacidosis (DKA) have a unique predisposition to this infection (Ibrahim et al., Clinical Mycology, Dismukes et al., eds. New York, N.Y.: Oxford University Press, pp. 241-251 (2003) Spellberg et al., Clin Microbiol Rev. 18(3):556-569 (2005)). Unfortunately, despite surgical debridement and first-line antifungal therapy, the overall mortality of mucormycosis remains unacceptably high, and survivors are typically left with considerable disfigurement from the infection and surgery (Spellberg (2005) supra; Gleissner et al., Leuk Lymphoma 45(7):1351-1360 (2004)).
[0123]A hallmark of mucormycosis is the presence of extensive a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
| Antigenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com