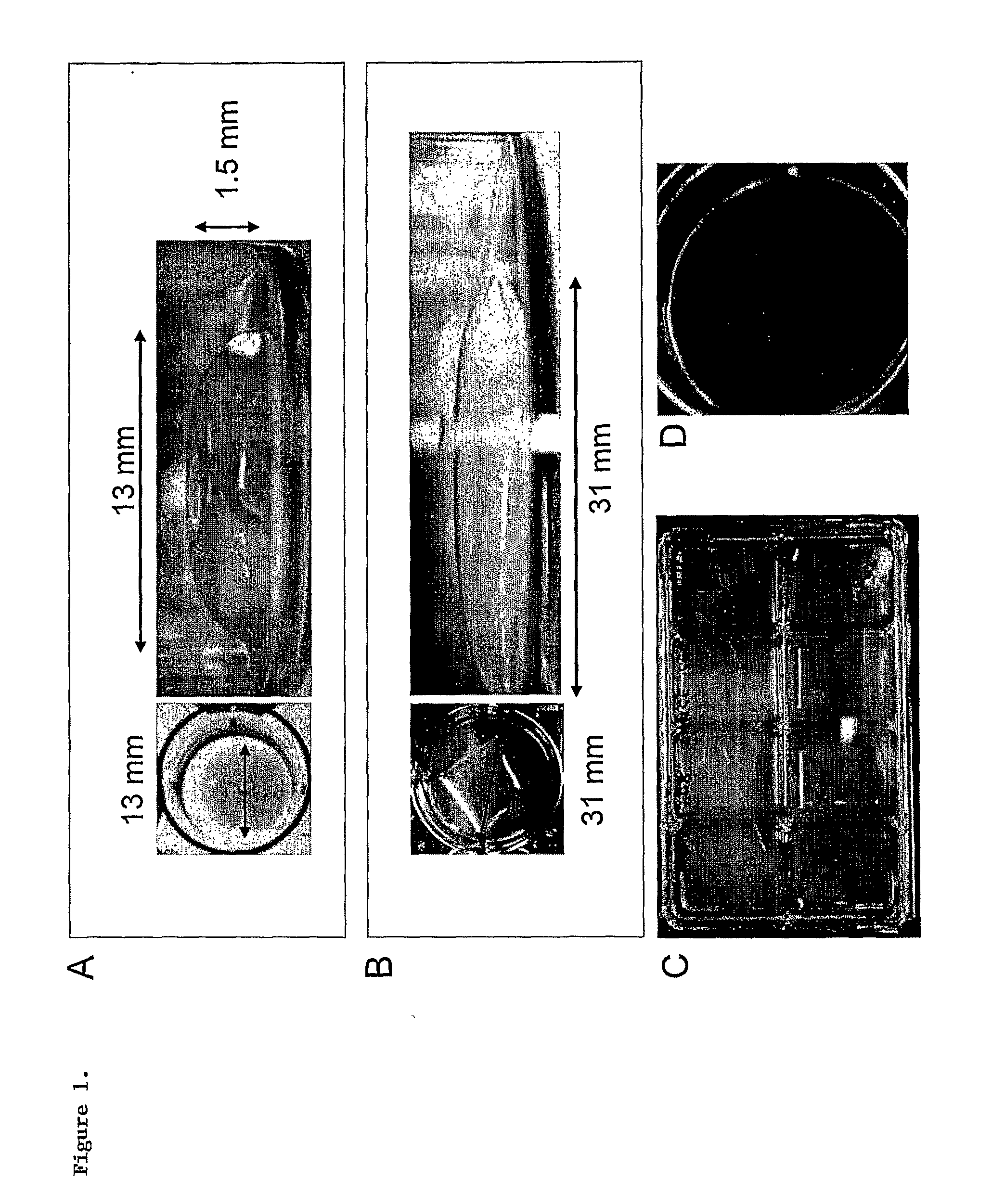
Skin equivalent culture
a technology of skin and composition, applied in the field of skin equivalent culture, can solve the problems of concave rather than concentric shape, loss of height of the construct, and doubt that the collagen will obtain its final structur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Discussion of Prior Art and the Present Invention
[0048]D1—Hansbrough J F, Morgan J L, Greenleaf G E and Bartel R. (1993). Composite grafts of Human keratinocytes grown on a polyglactin mesh-cultured fibroblast dermal substitute function as a bilayer skin replacement in full-thickness wounds on athymic mice. J. Burn Care and Rehab. 14 (5), 485-494.[0049]D2—Geesin et al, Regulation of Collagen Synthesis in human dermal fibroblasts in contracted collagen gels by ascorbic acid, growth factors, and inhibitors of lipid peroxidation. Experimental Cell Research 206, 283-290.[0050]D3—WO 03 / 41568 (University of New Jersey, Hewitt et. al) A Three dimensional matrix for producing living tissue equivalents.
Overview of ICX-SKN—Skin Equivalent Culture, According to the Invention:
[0051]ICX-SKN has been designed as an active cell therapy consisting of human dermal fibroblasts (HDFs) embedded in fibrin gels which are allowed to mature over several weeks. A key aspect of this maturation period is that...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com