Flexible Neural Localization Devices and Methods
a neural localization and flexible technology, applied in the field of flexible devices, can solve the problems of inability to accurately guide surgical procedures, risk damage to nerve tissue, and system variability, and achieve the effect of reducing the risk of nerve damag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
integrated embodiments
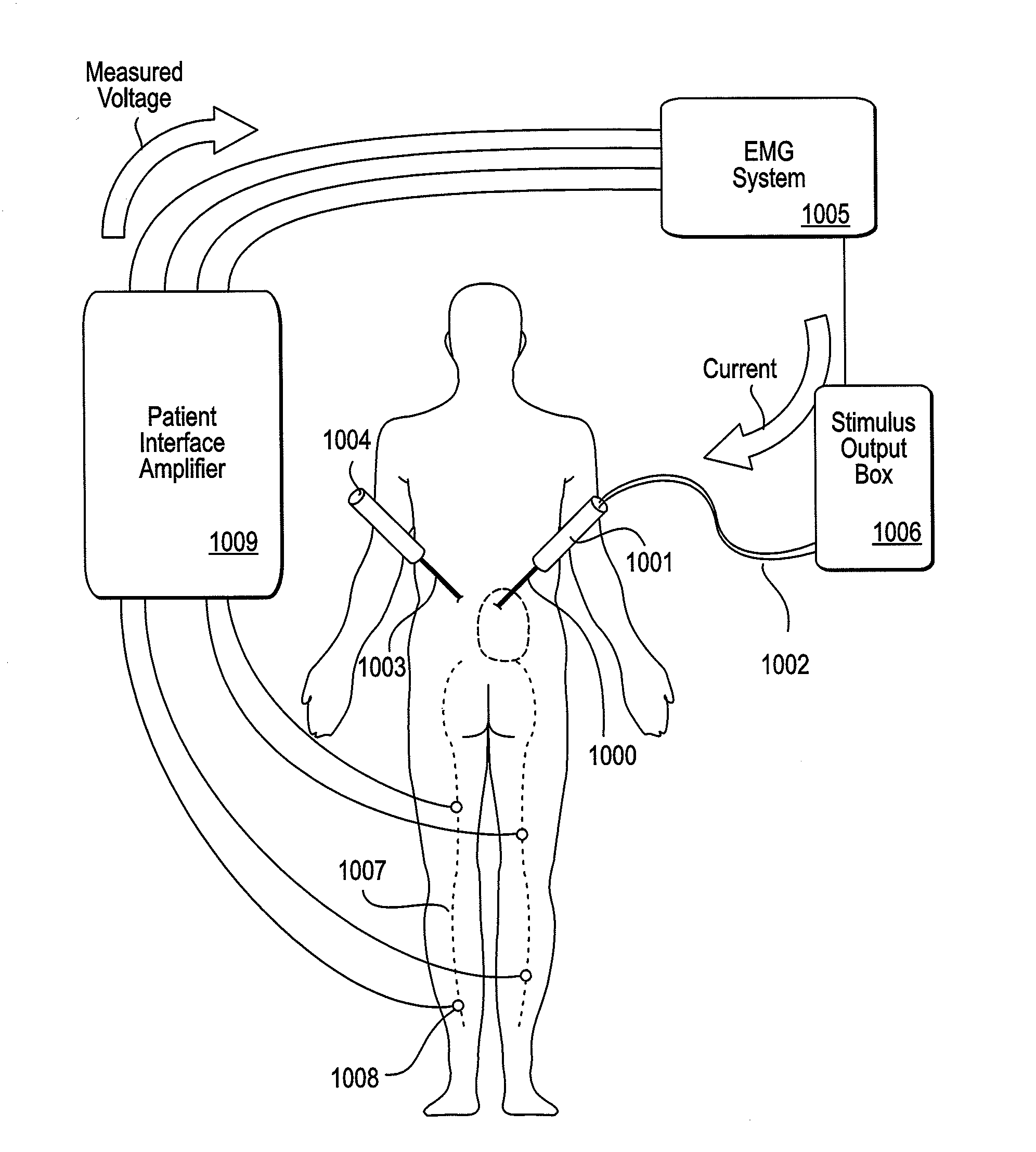
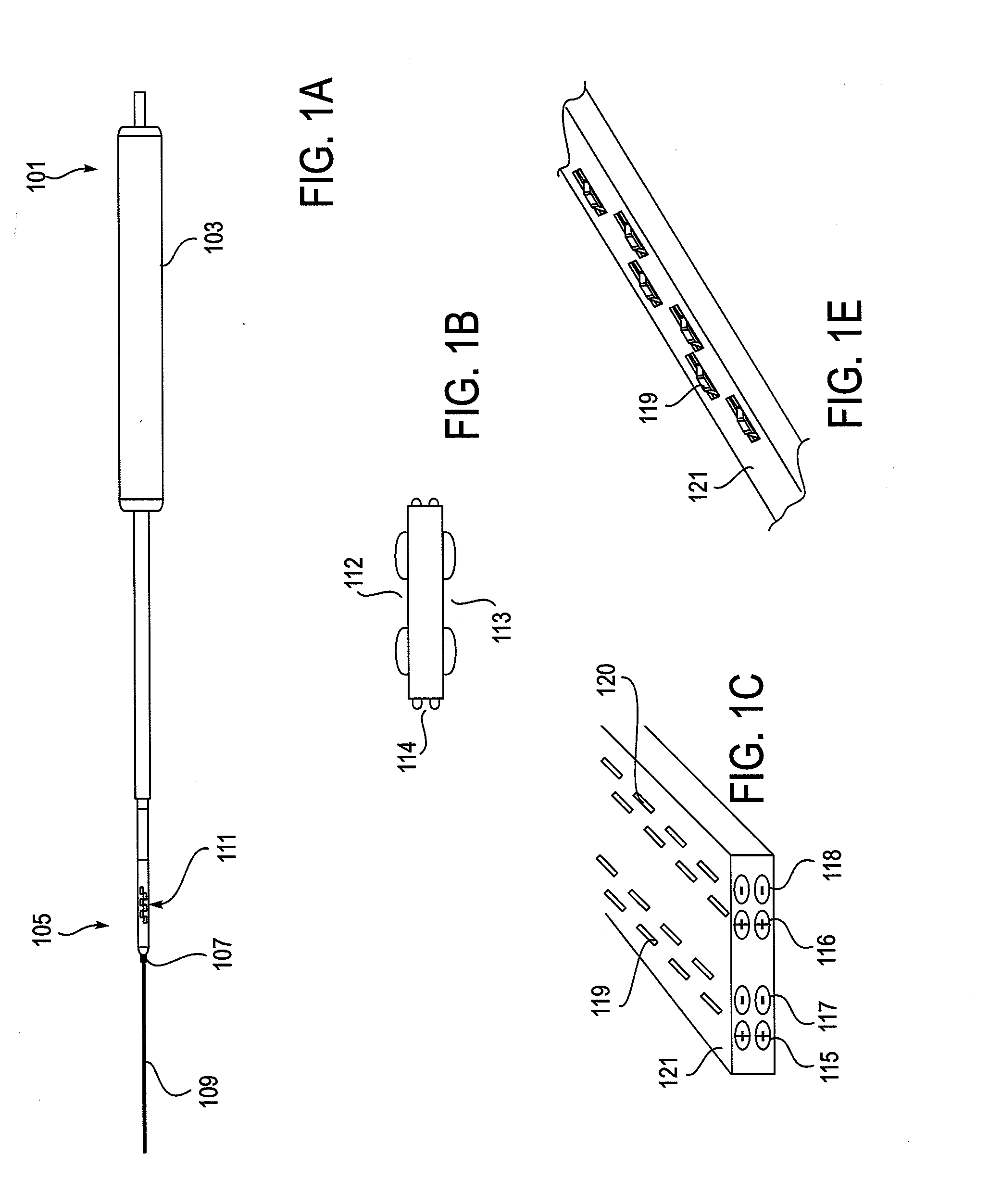
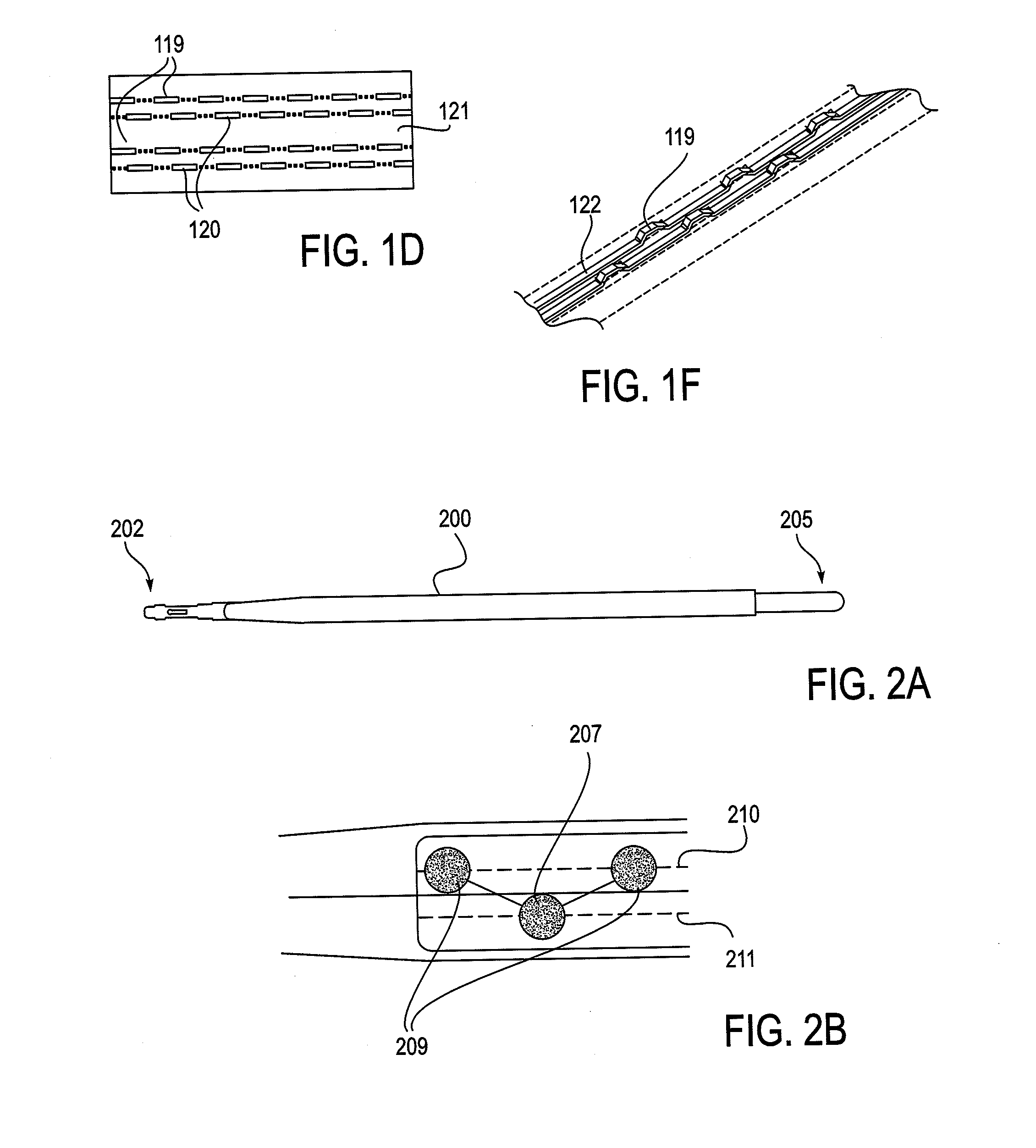
[0207]As mentioned above, the NLR device described herein may couple to one or more tissue modification devices. FIGS. 20A and 20B illustrate two examples of NLR devices 2000 that are configured to couple to tissue modification devices 2001. In FIG. 20A, the tissue modification device 2001 is an elongate flexible tissue removal device that includes tissue modification elements 2003 (e.g., “teeth”) on one side, and has a coupling element at the distal end 2005. The coupling element may be a guidewire coupler, or it may be configured to couple directly to an NLR device 2000. The NLR device 2000 (shown adjacent to the distal end of the tissue modification device in FIG. 20A) includes an opening or cavity into which the tissue modification device may at least partially enter and engage. In other variations, the proximal end of the NLR device is configured to couple to the distal end of the tissue modification device without entering the NLR device.
[0208]The NLR device in this example ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com