Viruses encoding mutant membrane protein
a virus and membrane protein technology, applied in the field of viruses encoding mutant membrane proteins, can solve the problems of obviating difficulties, unable to interrupt the electrophysiological measurement of nb protein based on the lipid bilayer system, and unable to meet the needs of patients, etc., to achieve the effect of altering the reading fram
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
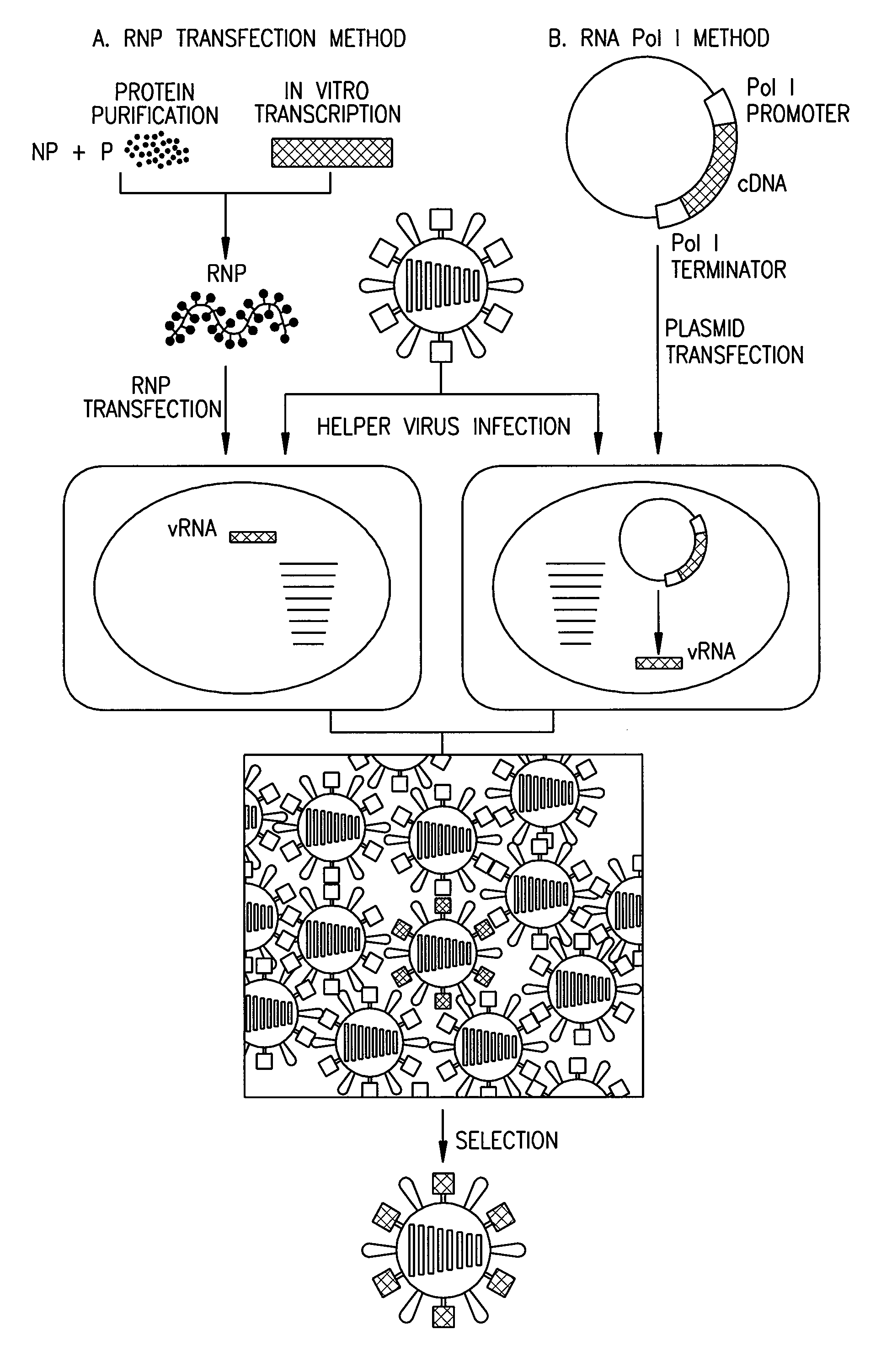
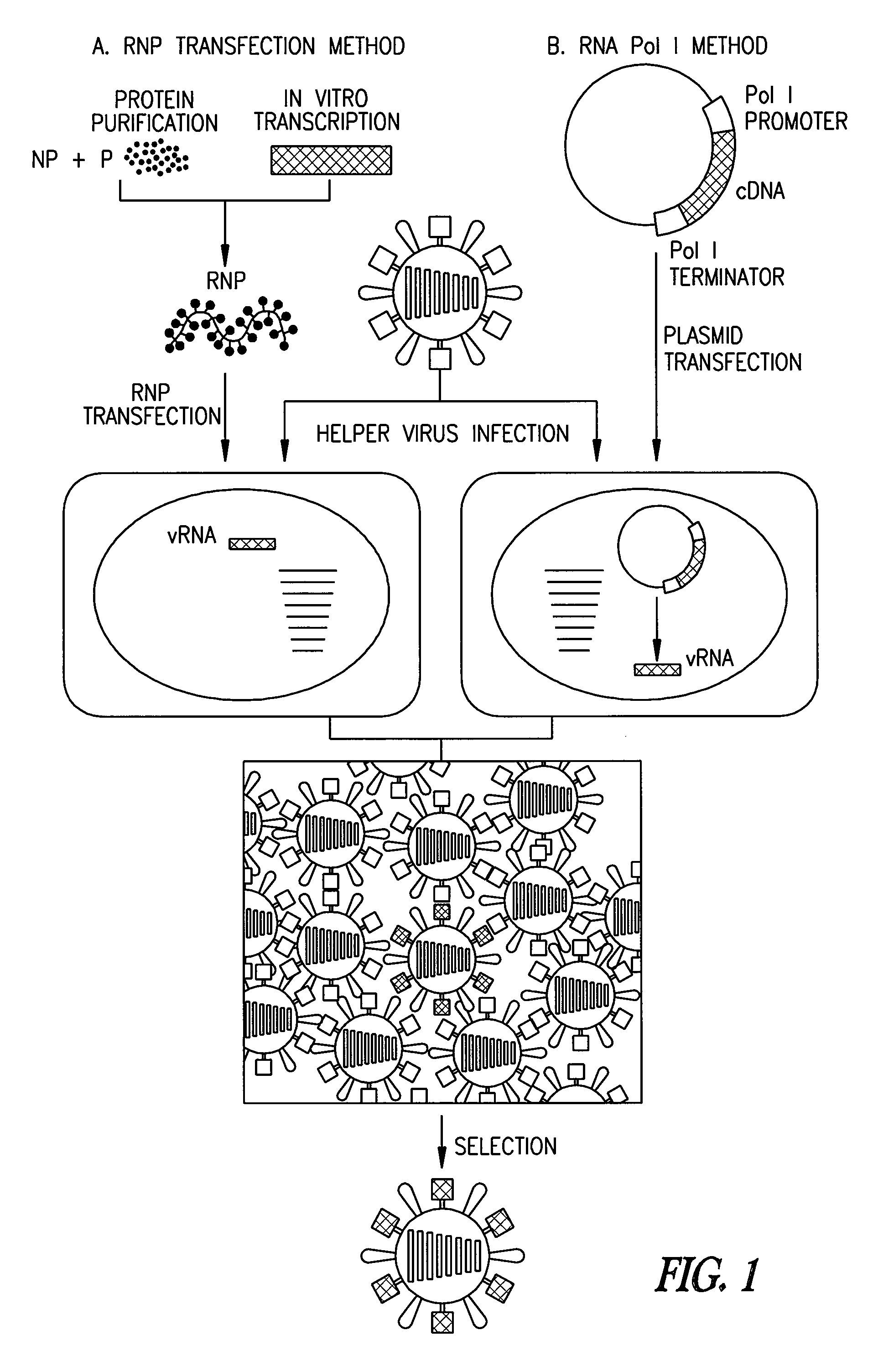
[0076]Cells and viruses. 293T human embryonic kidney cells and Madin-Darby canine kidney cells (MDCK) were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum and in modified Eagle's medium (MEM) containing 5% newborn calf serum, respectively. All cells were maintained at 37° C. in 5% CO2. Influenza viruses A / WSN / 33 (H1N1) and A / PR / 8 / 34 (H1N1) were propagated in 10-day-old eggs.
[0077]Construction of plasmids. To generate RNA polymerase I constructs, cloned cDNAs derived from A / WSN / 33 or A / PR / 8 / 34 viral RNA were introduced between the promoter and terminator sequences of RNA polymerase I. Briefly, the cloned cDNAs were amplified by PCR with primers containing BsmBI sites, digested with BsmBI, and cloned into the BsmBI sites of the pHH21 vector which contains the human RNA polymerase I promoter and the mouse RNA polymerase I terminator, separated by BsmBI sites (FIG. 2). The PB2, PB1, PA, HA, NP, NA, M, and NS genes of the ...
example 2
Materials and Methods
[0092]Cells, viruses, and antibodies. 293T human embryonic kidney cells and Madin-Darby canine kidney (MDCK) cells were maintained in DMEM supplemented with 10% fetal calf serum and in MEM containing 5% newborn calf serum, respectively. The 293T cell line is a derivative of the 293 line, into which the gene for the simian virus 40 T antigen was inserted (DuBridge et al., 1987). All cells were maintained at 37° C. in 5% CO2. B / Lee / 40 and its mutant viruses were propagated in 10-day-old embryonated chicken eggs. The viruses were purified from allantoic fluid by differential centrifugation and sedimentation through a 10-50% sucrose gradient. An anti-NB rabbit serum was generated against synthesized peptide NKRDDISTPRAGVD (SEQ ID NO:9; amino acid residues 70-83 of NB protein) coupled to keyhole limpet hemocyanin.
[0093]Construction of plasmids. The cDNAs of B / Lee / 40 viruses were synthesized by reverse transcription of viral RNA with an oligonucleotide complementary t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| hydrophobic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com