Droplet Libraries
a technology of droplets and libraries, applied in the field of droplets, can solve the problems of high detrimental, surface adsorption of reactants, and the limit of the smallest volume of reagents that can effectively be used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0277]Example 1 shows methods of surfactant syntheses.
[0278]Below of the reaction scheme for creating the surfactants utilized in stabilizing the droplet libraries provided by the instant invention.
Reagent Table is as follows:
Other (density,purity, safety,NameMWMoles / Equiv.Amount usedmisc.)Krytox FSH65001.54 mmol (1 eq)10.0 gOxalyl126.9315.4 mmol1.3 mLd = 1.5 g / mL;Chloride(10 eq)(1.95 g)b.p. 62-65° C.HFE 7100250.0650 mLb.p. 61° C.
[0279]The procedure includes, adding 10.0 g (1.54 mmol; 1 eq) of Krytox acid FSH in 50 mL HFE 7100 (not anhydrous) at right under Ar was added 1.95 g (15.4 mmol; 10 eq) of Oxalyl Chloride dropwise. Stirred 10 min, then the reaction was warmed to gentle reflux (note boiling points of solvent and reagent). Some bubbling was noted, even before reaction had reached reflux. Continued overnight.
[0280]The following day, the reaction was very slightly cloudy, and contained a very small amount of a yellow solid. Cooled, HFE and excess Oxalyl chloride evaporated. Res...
example 2
[0292]Example 2 shows PEG-Amine derived fluorosurfactant syntheses
[0293]A PEG-amine derived fluorosurfactant can be made by the following process: 10.0 g of Kytox 157 FSH (PFPE, 6500 g / mol, 0.00154 mole) was dissolved in 25.0-mL of FC-3283 (521 g / mol, 45.5 g, 0.0873 mole). 0.567 g PEG 600 Diamine (566.7 g / mol, 0.001 mole, 0.65 mol eq.) was dissolved in 10.0-mL of THF (72.11 g / mol, 8.9 g, 0.1234 mole). The resulting solutions were then combined and emulsified. The resulting emulsion was spun on a BUCHI rota-vap. at ˜75% for ˜20 hours. The crude reaction mixture was then placed in centrifuge tubes with equal volumes of DI H2O, emulsified and centrifuged at 15,000 rpm for 15-minutes. Once the emulsion was broken, the oil layer was extracted, dried with anhydrous sodium sulfate and filtered over a 0.45-um disposable nylon filter. The filtered oil was then evaporated on a BUCHI rota-vap. model R-200 fitted with a B-490 water bath for =2-hours at 70° C.
[0294]The procedure is depicted in S...
example 3
Primer Library Generation
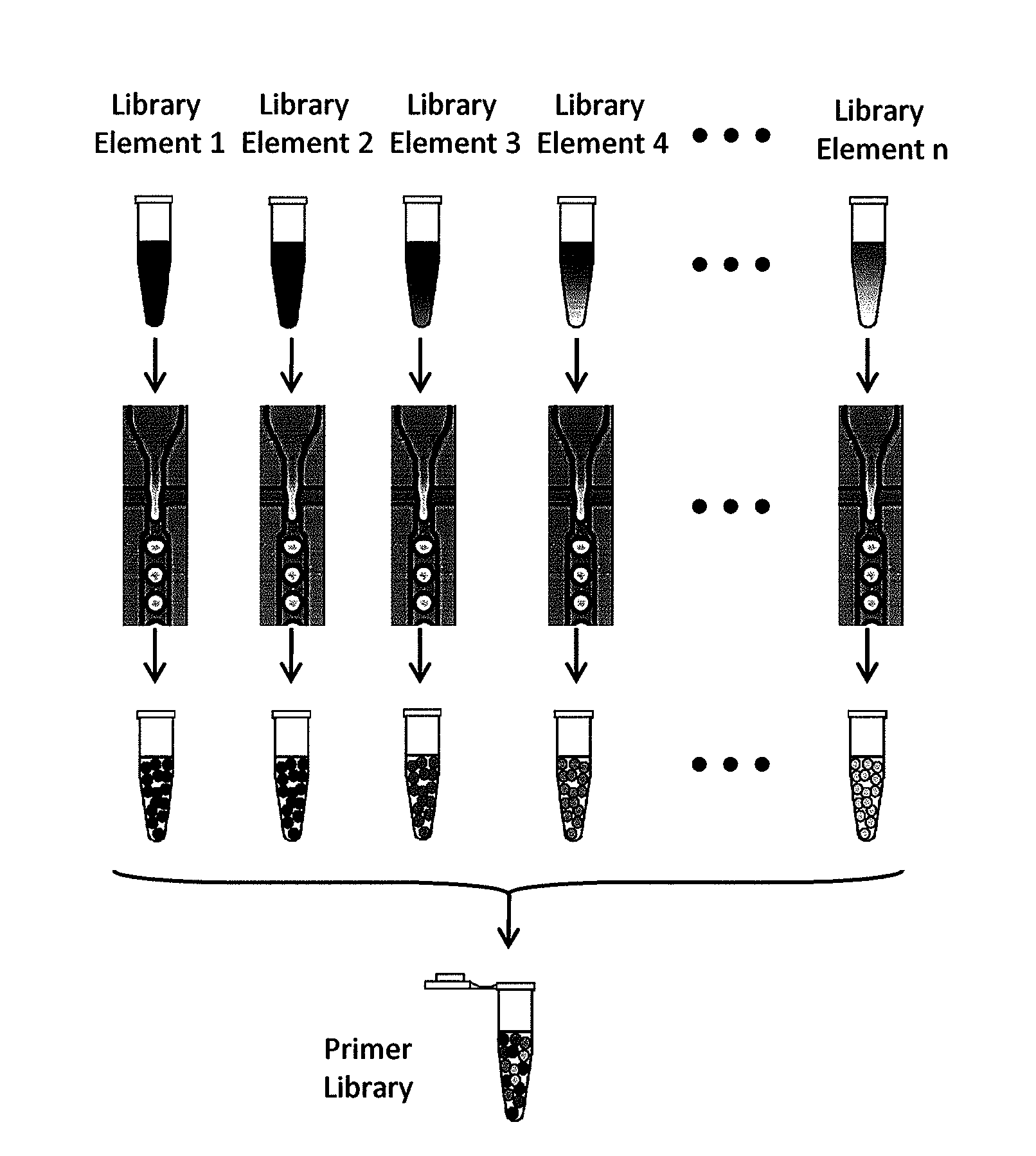
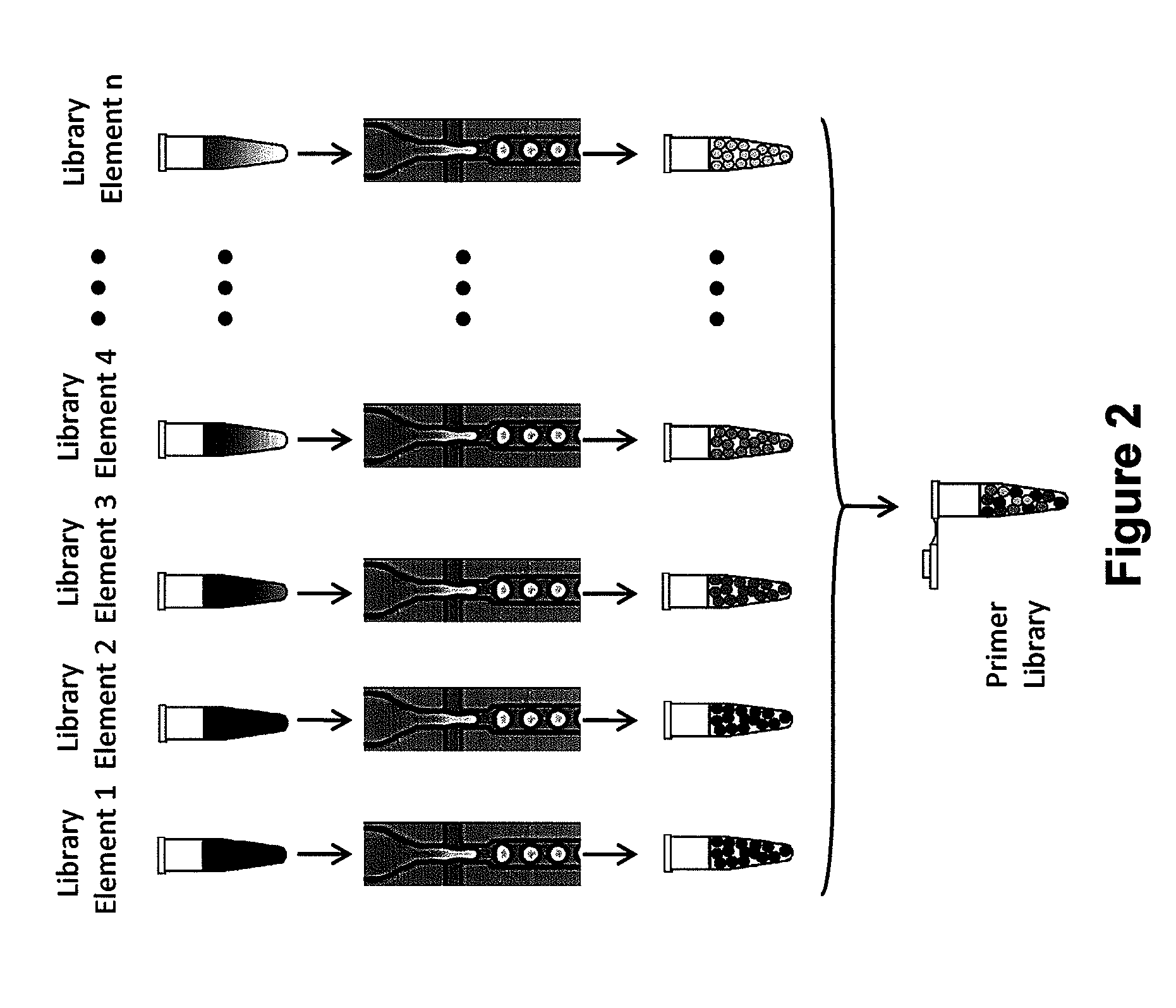
[0296]The primer droplet library generation is a Type IV library generation. FIG. 6 shows a schematic of the primer library generation. Step 1 of the library formation is to design primers for loci of interest. There are no constraints on primer design associated with traditional multiplex PCR. Step 2 requires synthesis of the primer pairs using standard oligo synthesis. After the library elements are created, the primer pairs are reformatted as droplets, where only one primer pair present in each droplet. Each droplet contains multiple copies of the single primer pair directed to a single target of interest. After the droplets for each type of primer pair is created, the emulsions are pooled as primer library. The droplet stability prevents cross-contamination of primer pairs.
[0297]Primer Library for Genome Selection
[0298]A pooled primer library can be placed onto a microfluidic device as provided by the instant invention. Each primer library droplet follow...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com