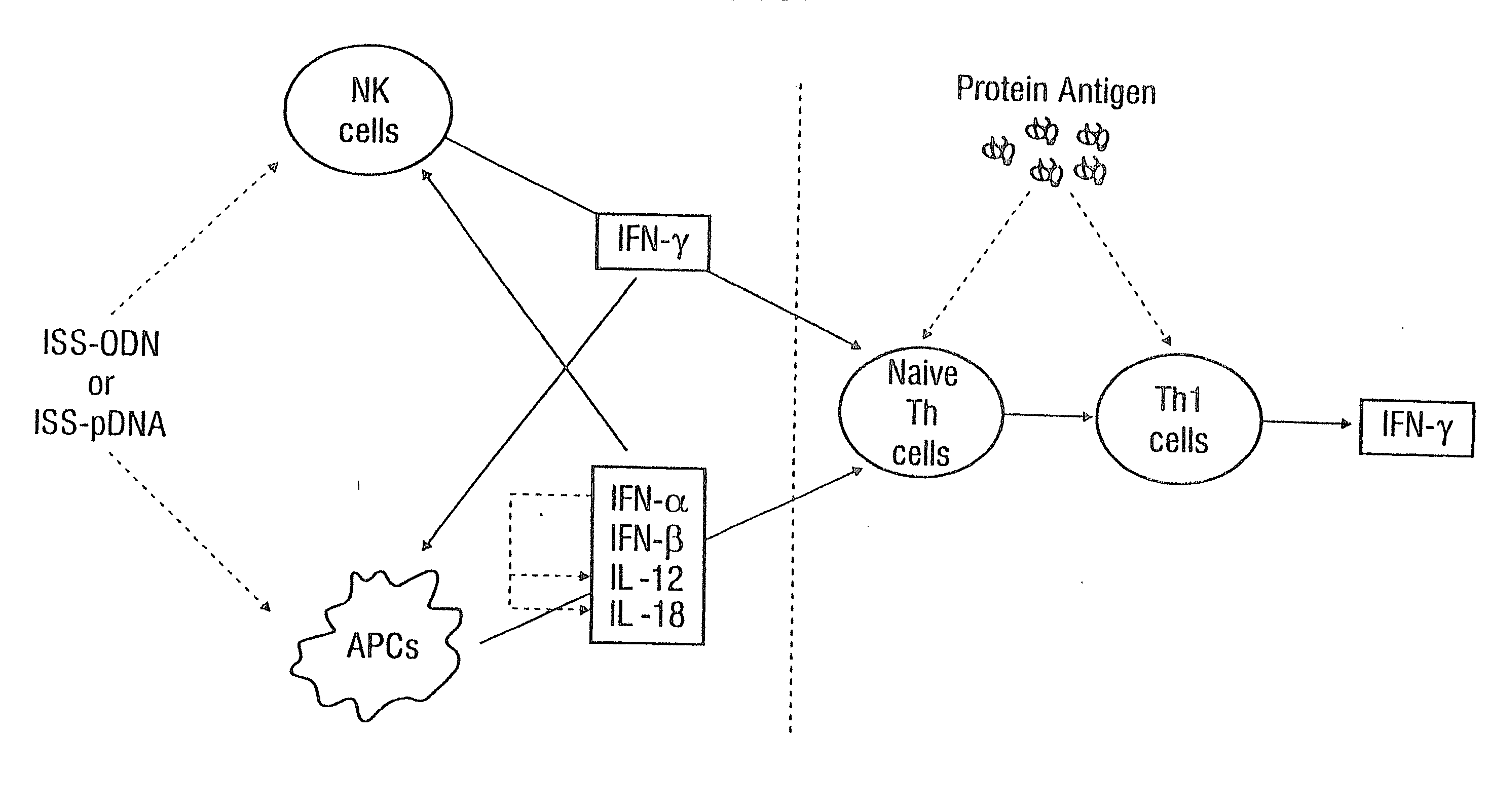
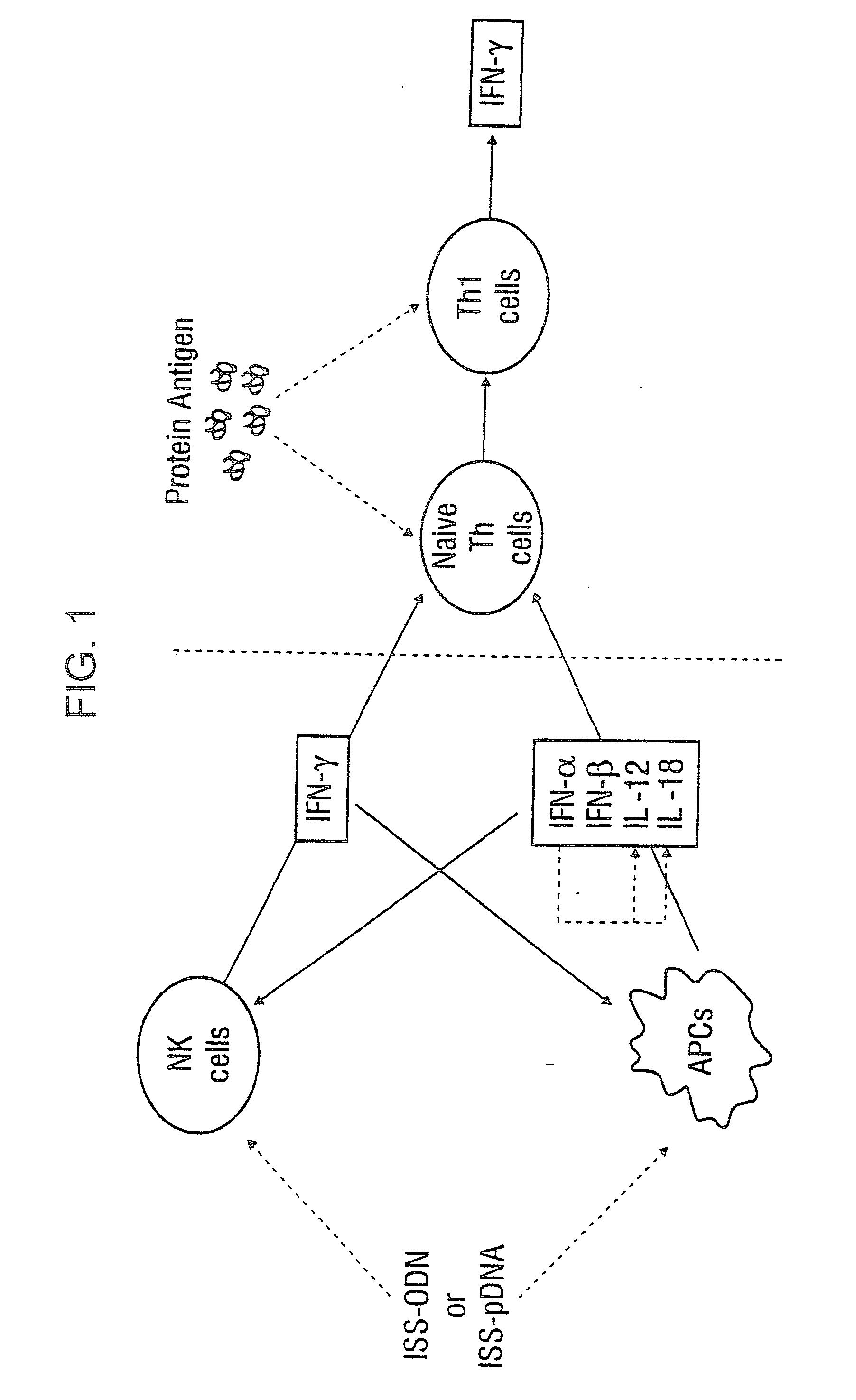
Immunization-free methods for treating antigen-stimulated inflammation in a mammalian host and shifting the host's antigen immune responsiveness to a th1 phenotype
a technology of immune responsiveness and immunity, applied in the direction of antibody medical ingredients, drug compositions, immunological disorders, etc., can solve the problems of ineffective long-term cellular immunity and inflammation damage of host tissues, and canonical immunization does not effectively stimulate long-term cellular immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Murine Model for the Airway Hyperreactivity of Allergic Asthma
[0103]Sensitizing-antigen challenged mice of different strains model the airway hyperreactivity seen in allergic asthma. Suitable murine strains for use in modeling the disease include Balb / c mice (which are biased genetically toward the Th2 phenotype and produce enhanced concentrations of IL-4 and IL-5 in response to antigen challenge to CD4+ lymphocytes), C57BL / 6 mice (which are IL-5 deficient, for detailed study of IL-5 induced tissue damage in asthma) and W / Wv mice (which are mast cell deficient, for detailed study of mast cell activation in asthma).
[0104]Disease modeling mice are conveniently prepared by intraperitoneal or subcutaneous injection of a model sensitizing antigen, ovalbumin (“OVA”) in carrier (e.g., sterile saline or a carrier with adjuvant, such as alum), followed by antigen challenge with aerosolized antigen. For example, mice may be immunized with 25 μg OVA by subcutaneous injection (with or without a...
example ii
Reduction of Eosinophil Accumulation in Lung Tissue in a Murine Asthma Model by Administration of ISS-ODN
[0106]BALB / c mice, 6-10 weeks of age, were prepared as models of allergic asthma as described in Example I (subcutaneous injection of OVA followed by antigen challenge at a concentration of 50 mg OVA / ml PBS). Prior to each inhalation with OVA according to this scheme, sets of 8 mice each were treated as described in the Table below. Control mice were antigen challenged but untreated and naive mice were not challenged with antigen. All ISS doses were 100 μg per administration. Dexamethasone (a conventional steroidal anti-inflammatory used in the treatment of asthma) doses were 5 mg / kg / mouse. Priming doses of antigen were 25 μg OVA adsorbed to alum in 0.2 ml phosphate buffered saline (PBS). Challenge doses of antigen were 10 ml of 50 mg OVA / ml PBS. 1N=intranasal; IP=intraperitoneal; SC=subcutaneous and N / A=not applicable.
Set #Material ReceivedRoute and Timing1Naive mice (no antigen...
example iii
Antigen Dependent Reduction of Eosinophil Accumulation in Lung Tissue
[0114]To evaluate whether the eosinophil suppression demonstrated by the data in Example II is dependent upon immune stimulation by the ISS-ODN, mice were sensitized to OVA using a conventional, Th2 stimulatory adjuvant (alum), treated with ISS-ODN or a control, and measured for eosinophil suppression before ISS-ODN stimulation of the mouse immune system would be expected to occur.
[0115]More specifically, groups of four mice were immunized with 25 μg OVA in 1 mg alum by subcutaneous injection on days 1, 7, 14 and 21. This immunization protocol is known to stimulate a Th2 type response to the antigen in mice. On day 27, one group of animals received 100 μg of the DY1018 ISS-ODN described in Example I by intraperitoneal administration. A control group received the mutant DY1019 M-ISS-ODN described in Example I by the same route.
[0116]On day 28, the animals in each group received 10 mg OVA / ml phosphate buffered saline...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com