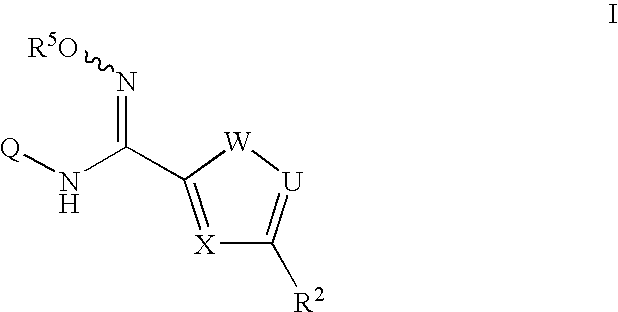
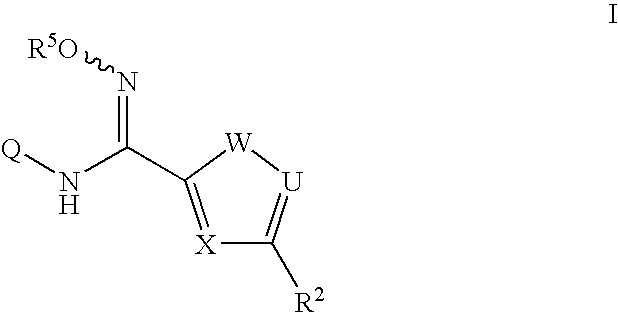
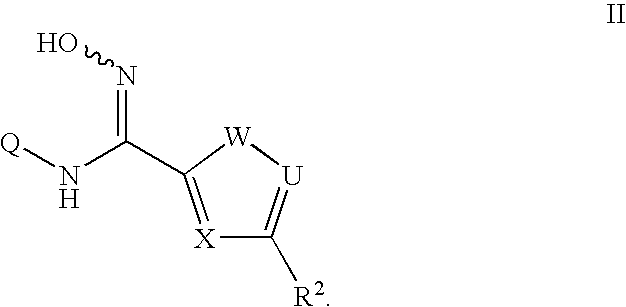
N-hydroxyamidinoheterocycles as modulators of indoleamine 2,3-dioxygenase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
N-(3-Chloro-4-fluorophenyl)-N′-hydroxy-3-(3-morpholin-1-ylpropoxy)isoxazole-5-carboximidamide
[0206]
Step A: Methyl 3-(3-bromopropoxy)isoxazole-5-carboxylate
[0207]
[0208]A solution of methyl 3-hydroxyisoxazole-5-carboxylate (3.5 g, 24 mmol) in NAN-dimethylformamide (20 mL) was treated with potassium carbonate (6.8 g, 49 mmol) and 1,3-dibromopropane (2.5 mL, 24 mmol) was added and the mixture was stirred at 40° C. for 1 h. The reaction was diluted with water and extracted with ethyl acetate three times. The combined extracts were dried with magnesium sulfate, filtered and concentrated in vacuo. The crude residue was purified by flash chromatography (0-70% ethyl acetate / hexanes) to give the desired product (1.6 g, 25%). MF=C8H11BrNO4; LCMS calculated for C8H11BrNO4 (M+H)+: m / z=264.
Step B: 3-(3-Bromopropoxy)isoxazole-5-carboxylic acid
[0209]
[0210]A solution of methyl 3-(3-bromopropoxy)isoxazole-5-carboxylate (250 mg, 0.93 mmol) in tetrahydrofuran (1.5 mL) was treated with 1 N aqueous sodiu...
example 2
N-(3-Chloro-4-fluorophenyl)-N′-hydroxy-3-(3-pyrrolidin-1-ylpropoxy)isoxazole-5-carboximidamide
[0216]
Step A: N-(3-Chloro-4-fluorophenyl)-3-(3-pyrrolidin-1-ylpropoxy)isoxazole-5-carboxamide
[0217]
[0218]A solution of 3-(3-bromopropoxy)-N-(3-chloro-4-fluorophenyl)isoxazole-5-carboxamide (38 mg, 0.10 mmol), and pyrrolidine (100 μL, 1.20 mmol) in tetrahydrofuran (1.5 mL) was stirred at room temperature for 4 h. The reaction was diluted with methanol and purified by preparative LCMS to give the desired product (34 mg, 92%). MF=C17H20ClFN3O3; LCMS calculated for C17H20ClFN3O3 (M+H)+: m / z=368.
Step B: N-(3-Chloro-4-fluorophenyl)-N′-hydroxy-3-(3-pyrrolidin-1-ylpropoxy)isoxazole-5-carboximidamide
[0219]This material was prepared according to the procedure of Example 1, Step D, using N-(3-chloro-4-fluorophenyl)-3-(3-pyrrolidin-1-ylpropoxy)isoxazole-5-carboxamide as the starting material. (24 mg, 66%). MF=C17H21ClFN4O3; LCMS calculated for C17H21ClFN4O3 (M+H)+: m / z=383.
example 3
N-(3-Chloro-4-fluorophenyl)-N′-hydroxy-3-morpholin-ylisoxazole-5-carboximidamide
[0220]
Step A: 3-Bromo-N-(3-chloro-4-fluorophenyl)isoxazole-5-carboxamide
[0221]
[0222]This material was prepared according to the procedure of Example 1, Step C, using 3-bromoisoxazole-5-carboxylic acid and 3-chloro-4-fluoro aniline as starting materials. MF=C10H5BrClFN2O2; LCMS calculated for C10H5BrClFN2O2 (M+H)+: m / z=319.
Step B: N-(3-Chloro-4-fluorophenyl)-3-morpholin-4-ylisoxazole-5-carboxamide
[0223]
[0224]A solution of 3-bromo-N-(3-chloro-4-fluorophenyl)isoxazole-5-carboxamide (7.5 mg, 0.024 mmol) in morpholine (1.0 mL) was heated at 180° C. in the microwave for 15 minutes. Purification by preparative LCMS (pH 10 buffer) gave the desired product (3.2 mg, 42%). MF=C14H14ClFN3O3; LCMS calculated for C14H14ClFN3O3 (M+H)+: m / z=326.
Step C: N-(3-Chloro-4-fluorophenyl)-N′-hydroxy-3-morpholin-ylisoxazole-5-carboximidamide
[0225]This material was prepared according to the procedure of Example 1, Step D, using N-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
| Electrical conductance | aaaaa | aaaaa |
| Electrical conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com