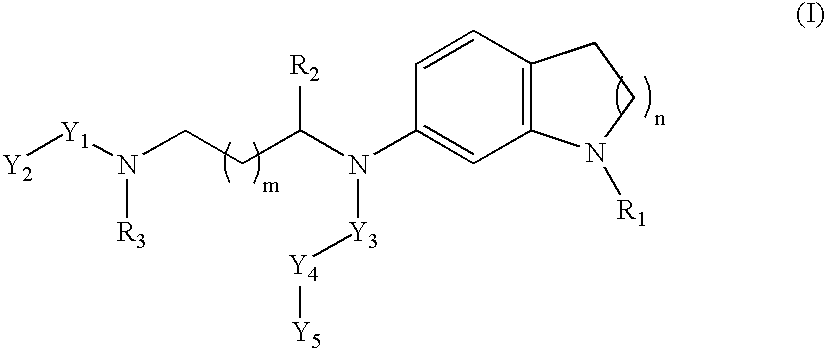
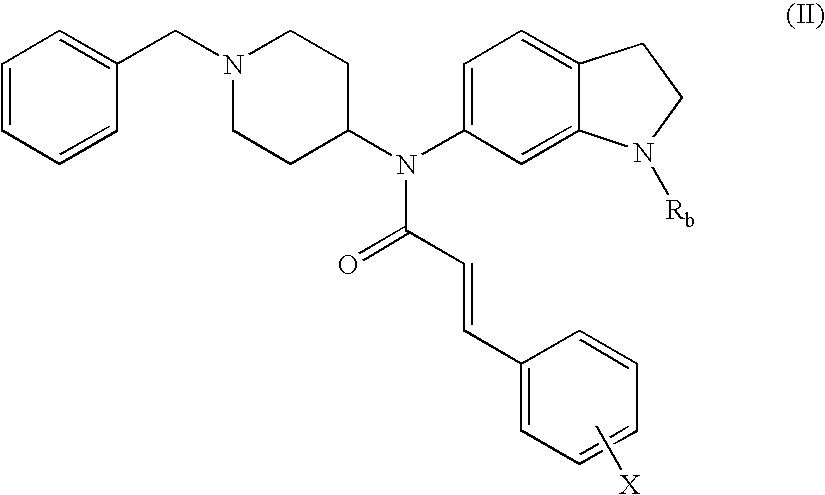
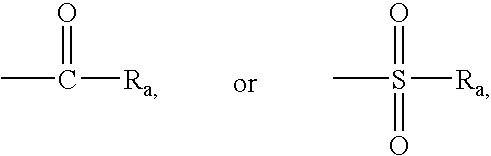Non-peptidic npy y2 receptor inhibitors
a technology of npy y2 receptor and inhibitor, which is applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problem of limited therapeutic potential of these compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0106]
trans-N-(1-Acetyl-2,3-dihydro-1H-indol-6-yl)-N-(1-benzyl-piperidin-4-yl)-3-phenyl-acrylamide
[0107]To a solution of 1-[6-(1-benzyl-piperidin-4-ylamino)-2,3-dihydro-indol-1-yl]-ethanone (250 mg, 0.72 mmol) in CH2Cl2 (10 mL) was added cinnamoyl chloride (160 mg, 0.93 mmol) and triethylamine (TEA, 0.30 mL, 2.2 mmol). The mixture was stirred at 25° C. for 16 h. After concentration, the residue was purified by preparative TLC (PTLC, 20% EtOAc / CH2Cl2) to provide 290 mg (85%) of the desired product. 1H NMR (500 MHz, CDCl3): 8.05 (s, 1H), 7.62 (d, J=15.5 Hz, 1H), 7.32-7.20 (m, 10H), 7.17 (d, J=7.9 Hz, 1H), 6.74 (dd, J=6.8, 1.5 Hz, 1H), 6.15 (d, J=15.5 Hz, 1H), 4.80-4.69 (m, 1H), 4.20-4.09 (m, 3H), 3.51-3.45 (m, 1H), 3.31-3.20 (m, 2H), 2.95-2.82 (m, 2H), 2.23 (s, 3H), 2.19-2.08 (m, 2H), 1.91-1.71 (m, 2H). MS: exact mass calculated for C31H33N3O2, 479.26; m / z found, 480.3 [M+H]+.
example 2
[0108]
trans-N-(1-Benzyl-piperidin-4-yl)-N-(2,3-dihydro-1H-indol-6-yl)-3-phenyl-acrylamide
Step A. 6-Amino-2,3-dihydro-indole-1-carboxylic acid tert-butyl ester
[0109]To a mixture of 6-nitroindoline (2 mmol) and TEA (2.2 mmol) in CH2Cl2 (20 mL) was added di-tert-butyl dicarbonate (2 mmol). The mixture was stirred at 25° C. for 16 h. The mixture was washed with satd. aq. NaHCO3 (20 mL) and brine (20 mL), and then was dried and concentrated. The residue was dissolved in MeOH (4 mL), and FeCl3.6H2O (7 mg), Me2NNH2 (21 mmol), and charcoal (50 mg) were added. The resulting mixture was heated at reflux for 4 h. The mixture was filtered through diatomaceous earth and the filtrate was concentrated to obtain the desired compound in quantitative yield.
Step B. 6-(1-Benzyl-piperidin-4-ylamino)-2,3-dihydro-indole-1-carboxylic acid tert-butyl ester
[0110]1-Benzyl-4-piperidone (1 mmol) was stirred with 6-amino-2,3-dihydro-indole-1-carboxylic acid tert-butyl ester (1.2 mmol), Na(OAc)3BH (1.3 mmol) and ...
example 3
[0113]
trans-N-(1-Benzyl-piperidin-4-yl)-N-(1-formyl-2,3-dihydro-1H-indol-6-yl)-3-phenyl-acrylamide
[0114]To a solution of the compound prepared in Example 2 (24 mg, 0062 mmol) in acetonitrile (2 mL) was added formic acid (2.2 μL, 0.060 mmol) and 1,1′-carbonyldiimidazole (11 mg, 0.060 mmol). The mixture was stirred at 25° C. for 16 h. After concentration, the residue was purified by PTLC (20% EtOAc / CH2Cl2) to give the title compound (10 mg, 39%). 1H NMR (500 MHz, CDCl3): 8.88 (s, 0.75H), 8.52 (s, 0.25H), 7.66 (d, J=15.6 Hz, 1H), 7.33-7.21 (m, 12H), 6.78 (dd, J=6.2, 1.7 Hz, 1H), 6.14-6.11 (m, 1H), 4.77-4.72 (m, 1H), 4.22-4.13 (m, 2H), 3.47-3.45 (m, 2H), 3.27-3.19 (m, 2H), 3.00-2.82 (br m, 2H), 2.17-2.13 (br m, 2H), 1.84-1.78 (m, 2H), 1.50-1.44 (m, 2H). MS: exact mass calculated for C30H31N3O2, 465.24; m / z found, 466.2 [M+H]+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com