Antibody immunization therapy for treatment of atherosclerosis
an antibody and atherosclerosis technology, applied in the field of new recombinant human antibodies, can solve the problems of efficacy and plasma half-lives reduction of murine antibodies, and achieve the effect of reducing the risk of allergic reactions and long plasma half-life of antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
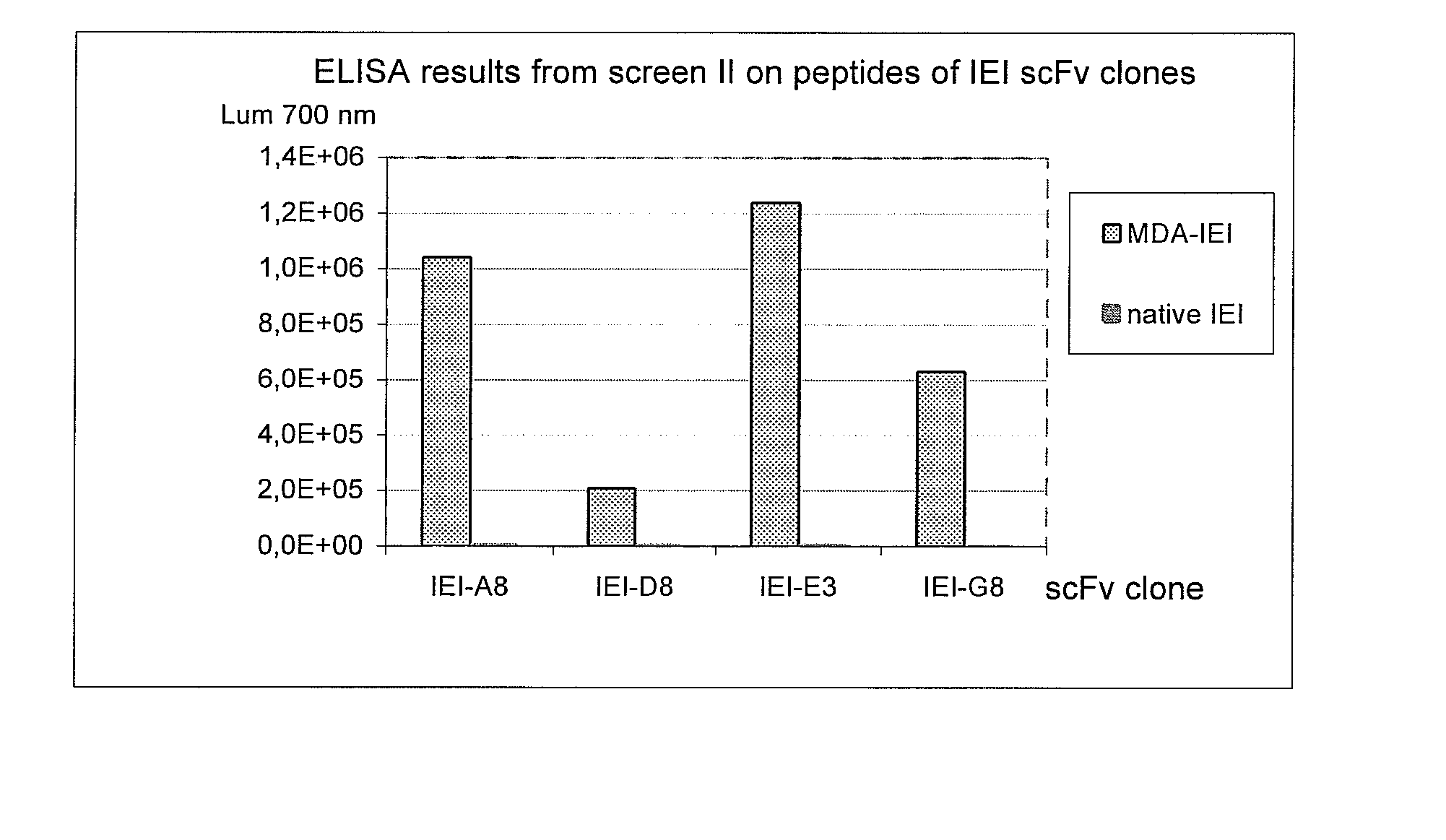
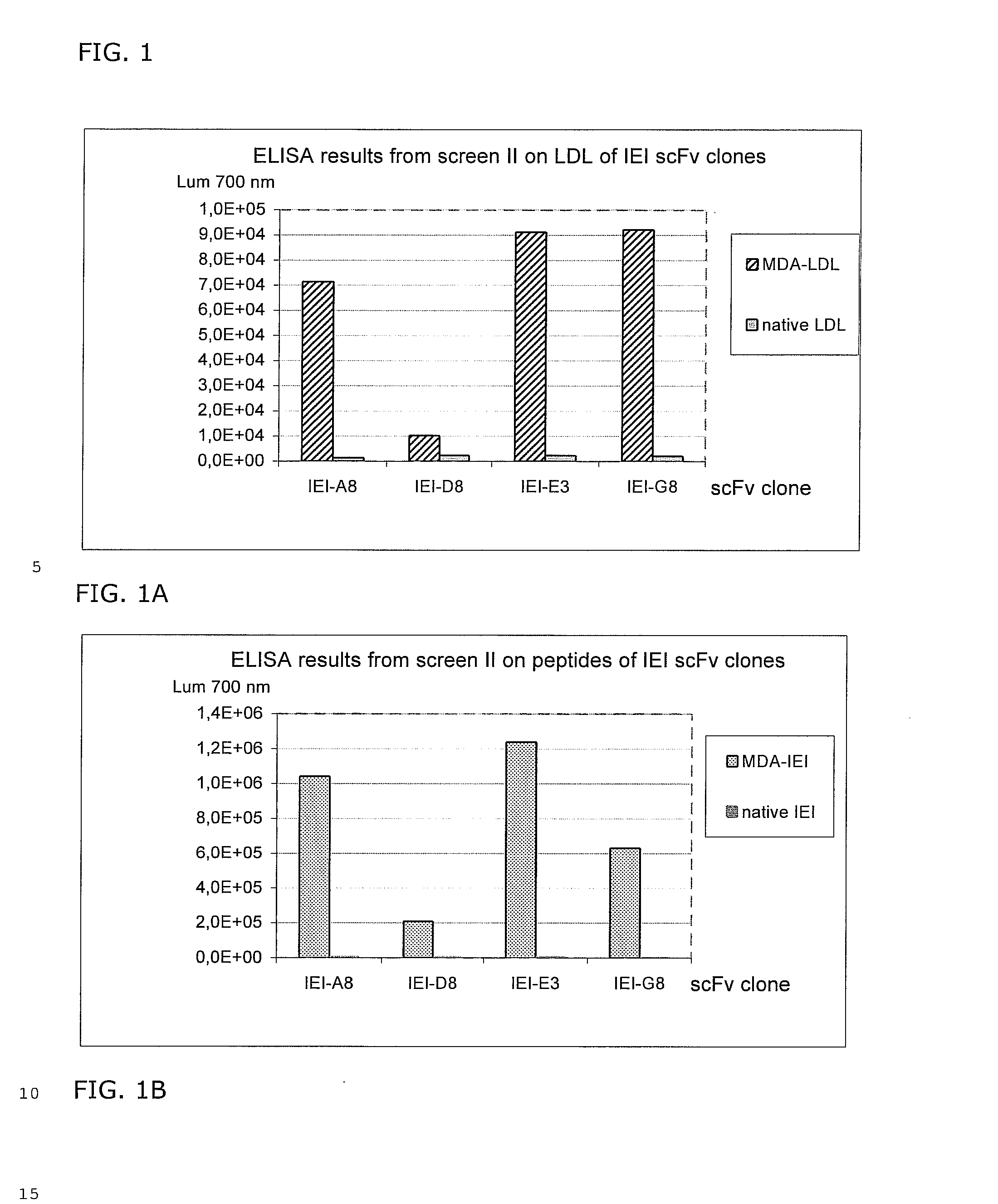
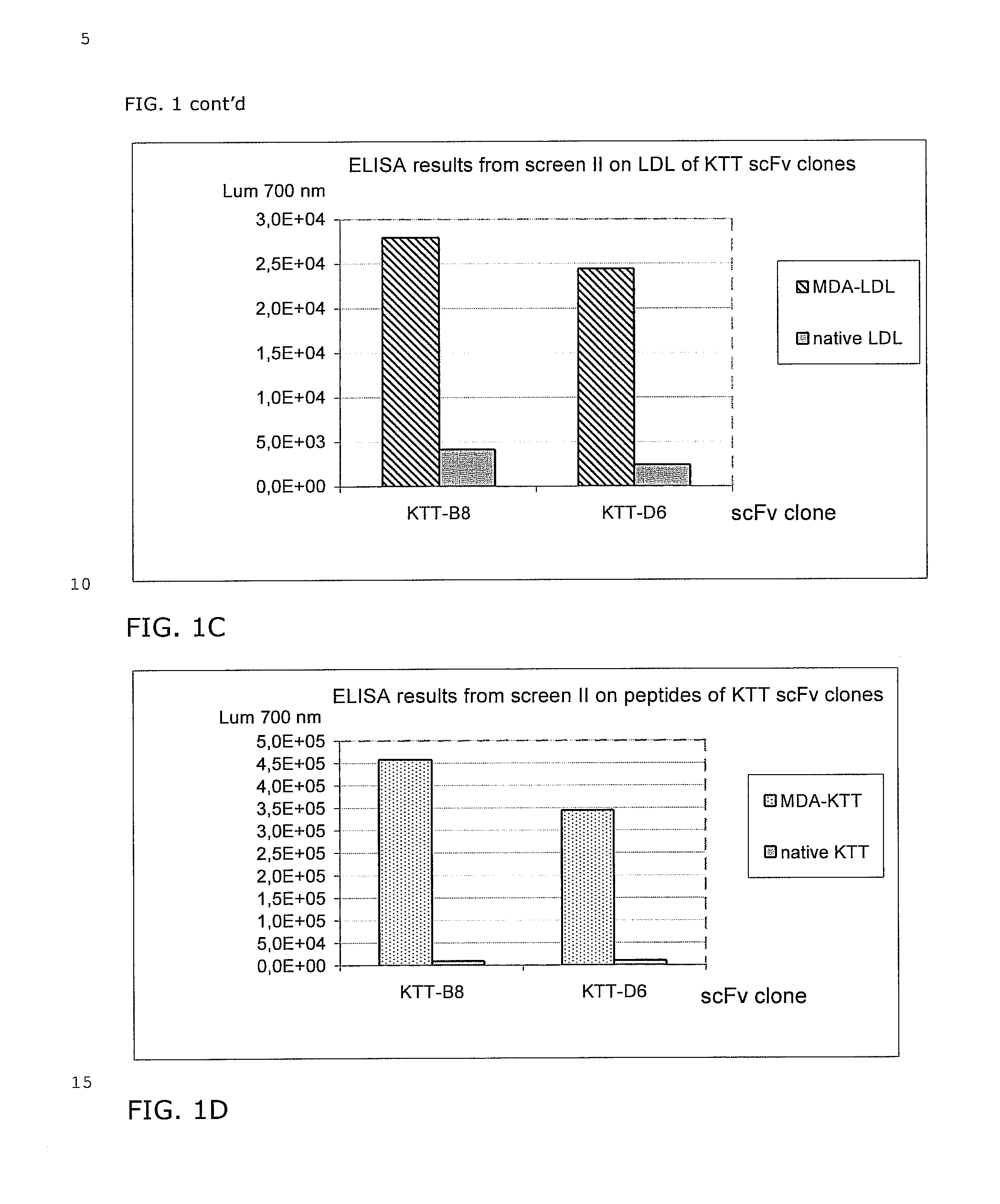
Selection of scFv Against MDA Modified Peptides IEIGL EGKGF EPTLE ALFGK (SEQ.ID NO: 70) (P45, Table 1) and KTTKQ SFDLS VKAQY KKNKH (SEQ. ID NO: 52) (P210, Table 1).
[0050] The target antigens were chemically modified to carry Malone-di-aldehyde (MDA) groups on lysines and histidines. The modified peptides were denoted IEI (P45) and KTT (P210).
[0051] Selections were performed using BioInvent's n-CoDeR™ scFv library for which the principle of construction and production have been described in Soderlind et al. 2000, Nature BioTechnology. 18, 852-856. The library contains approximately 2×1010 independent clones and a 2000 fold excess of clones were used as input for each selection. Selections were performed in three rounds. In selection round 1, Immunotubes (NUNC maxisorb™ 444202) were coated with 1.2 ml of 20 μg / ml MDA-modified target peptides in PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4) with end over end agitation at +4° C. over night. The tubes were then blocked w...
example 2
Transfer of Genes Encoding the Variable Parts of Selected scFv to Full Length Human IgG1 Vestors
[0062] Bacteria containing scFv clones to be converted to Ig-format were grown over night in LB supplemented with 100 μg / ml ampicillin. Plasmid DNA was prepared from over night cultures using the Quantum Prep, plasmid miniprep kit from Biorad (# 732-6100). The DNA concentration was estimated by measuring absorbance at 260 nm, and the DNA was diluted to a concentration of 2 ng / μl. VH and VL from the different scFv-plasmids were PCR amplified in order to supply these segments with restriction sites compatible with the expression vectors (see below). 5′ primers contain a BsmI and 3′ primers contain a BsiWI restriction enzyme cleavage site (shown in italics). 3′ primers also contained a splice donor site (shown in bold).
Primers for amplification of VH-segments:(SEQ. ID. NO: 13)5′VH:5′-GGTGTGCATTCCGAGGTGCAGCTGTTGGAG(SEQ. ID. NO: 14)3′VH:5′-GACGTACGACTCACCTGAGCTCACGGTGACCAGPrimers for amplif...
example 3
Stable Transfection of NSO Cells Expressing Antibodies Against MDA Modified Peptides from Apolipoprotein B-100
[0076] NSO cells (ECACC no. 85110503) were cultured in DMEM (cat nr 31966-021, Invitrogen) supplemented with 10% Fetal Bovine Serum (cat no. 12476-024, lot: 1128016, Invitrogen) and 1×NEAA (non-essential amino acids, cat no. 11140-053, Invitrogen). Cell cultures are maintained at 37° C. with 5% CO2 in humidified environment.
[0077] DNA constructs to be transfected were four constructs of IEI specific antibodies (IEI-A8, IEI-D8, IEI-E3, IEI-G8), two of KTT specific antibodies (KTT-B8, KTT-D6) and one control antibody (JFPA12). The day before transfection, the cells were trypsinized and counted, before plating them in a T-75 flask at 12×106 cells / flask. On the day of transfection, when the cells were 85-90% confluent, the cells were plated in 15 ml DMEM+1×NEAA+10% FBS (as above). For each flask of cells to be transfected, 35-40 μg of DNA were diluted into 1.9 ml of OPTI-MEM® ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com